Lawsone methyl ether
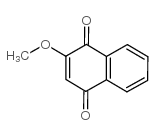
Lawsone methyl ether structure
|
Common Name | Lawsone methyl ether | ||
|---|---|---|---|---|
| CAS Number | 2348-82-5 | Molecular Weight | 188.17900 | |
| Density | 1.28g/cm3 | Boiling Point | 339.8ºC at 760mmHg | |
| Molecular Formula | C11H8O3 | Melting Point | 184-187ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | 152.7ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
2-Methoxy-1,4-naphthoquinone (MNQ) induces apoptosis of A549 lung adenocarcinoma cells via oxidation-triggered JNK and p38 MAPK signaling pathways.
Life Sci. 135 , 158-64, (2015) The compound 2-methoxy-1,4-naphthoquinone (MNQ) was previously shown to be cytotoxic against several cancer cell lines, but its mode of action is poorly understood. In this study, we aimed to explore the molecular mechanism of MNQ-induced cytotoxicity of A549... |
|
|
Anti-gastric adenocarcinoma activity of 2-Methoxy-1,4-naphthoquinone, an anti-Helicobacter pylori compound from Impatiens balsamina L.
Fitoterapia 83(8) , 1336-44, (2012) 2-Methoxy-1,4-naphthoquinone (MeONQ) from Impatiens balsamina L. exhibited strong anti-H. pylori activity in our previous study. In this study, we investigated the cytotoxicity of MeONQ against gastric adenocarcinoma (MKN45 cell line) and propose the relevant... |
|
|
Effects of the compounds 2-methoxynaphthoquinone, 2-propoxynaphthoquinone, and 2-isopropoxynaphthoquinone on ecdysone 20-monooxygenase activity.
Arch. Insect Biochem. Physiol. 66(1) , 45-52, (2007) The effects of the natural compound 2-methoxy-1,4-naphthoquinone, isolated from the leaves of Impatiens glandulifera and the synthetic compounds 2-propoxy-1,4-naphthoquinone and 2-isopropoxy-1,4-naphthoquinone on ecdysone 20-monooxygenase (E-20-M) activity we... |
|
|
Antipruritic dinaphthofuran-7,12-dione derivatives from the pericarp of Impatiens balsamina.
J. Nat. Prod. 61(9) , 1126-9, (1998) Dinaphthofuran-7,12-dione derivatives named balsaminones A (1) and B (2) were isolated from the pericarp of Impatiens balsamina L. together with the known compound 2-methoxy-1,4-naphthoquinone (3). Their structures were elucidated by spectral techniques. Thes... |
|
|
Isolation of an antimicrobial compound from Impatiens balsamina L. using bioassay-guided fractionation.
Phytother Res. 15(8) , 676-80, (2001) By using brine shrimp (Artemia salina) lethality test-guided fractionation, a single bioactive compound (LC(50)=26 ppm) was isolated from the 95% ethanol extract of the dried aerial parts of Impatiens balsamina L. and subsequently identified as 2-methoxy-1,4-... |
|
|
Antifungal activity of lawsone methyl ether in comparison with chlorhexidine.
J. Oral. Pathol. Med. 40(1) , 90-6, (2011) The aim of this study was to determine the antifungal activity of lawsone methyl ether mouthwash (LME) in comparison with chlorhexidine mouthwash (CHX) in vitro and in vivo.For in vitro study, each mouthwash preparation was added into the inoculum of Candida.... |
|
|
2-methoxy-1,4-naphthoquinone isolated from Impatiens balsamina in a screening program for activity to inhibit Wnt signaling.
J. Nat. Med. 65(1) , 234-6, (2011) A screening study using a luciferase assay to identify natural products which inhibit Wnt signaling was carried out. The bioassay-guided fractionation of aerial parts of a plant, Impatiens balsamina, led to the isolation of 2-methoxy-1,4-naphthoquinone (1) as... |
|
|
An antifungal naphthoquinone, xanthones and secoiridoids from Swertia calycina.
Planta Med. 61(4) , 362-4, (1995) A chemical and biological screening of 25 species of the Gentianaceae family has been undertaken. Both methanolic and dichloromethane extracts of Swertia calycina exhibited a strong antifungal activity against Cladosporium cucumerinum and Candida albicans. Th... |
|
|
General method for the high yield preparation of 2-(4-X-phenylene) amine-1, 4-naphthoquinones (X= ferrocenyl, OMe, Me, I, Cl, and NO2) from 2-methoxy-1, 4-naphthoquinone and investigation of H+ and Mg2+ catalysts with DFT calculations. Francisco AI, et al.
J. Mol. Struct. 891(1) , 228-32, (2008)
|