Effects of the compounds 2-methoxynaphthoquinone, 2-propoxynaphthoquinone, and 2-isopropoxynaphthoquinone on ecdysone 20-monooxygenase activity.
Martin J Mitchell, Aaron I Brescia, Stan L Smith, E David Morgan
Index: Arch. Insect Biochem. Physiol. 66(1) , 45-52, (2007)
Full Text: HTML
Abstract
The effects of the natural compound 2-methoxy-1,4-naphthoquinone, isolated from the leaves of Impatiens glandulifera and the synthetic compounds 2-propoxy-1,4-naphthoquinone and 2-isopropoxy-1,4-naphthoquinone on ecdysone 20-monooxygenase (E-20-M) activity were examined in three insect species. Homogenates of wandering stage third instar larvae of Drosophila melanogaster, or abdomens from adult female Aedes aegypti, or fat body or midgut from fifth instar larvae of Manduca sexta were incubated with radiolabelled ecdysone and increasing concentrations (from 1 x 10(-8) to 1 x 10(-3) M) of the three compounds. All three compounds were found to inhibit in a dose-dependent fashion the E-20-M activity in the three insect species. The concentration of these compounds required to elicit a 50% inhibition of this steroid hydroxylase activity in the three insect species examined ranged from approximately 3 x 10(-5) to 7 x 10(-4) M.(c) 2007 Wiley-Liss, Inc.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
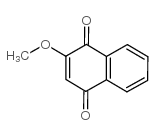 |
Lawsone methyl ether
CAS:2348-82-5 |
C11H8O3 |
|
2-Methoxy-1,4-naphthoquinone (MNQ) induces apoptosis of A549...
2015-08-15 [Life Sci. 135 , 158-64, (2015)] |
|
Anti-gastric adenocarcinoma activity of 2-Methoxy-1,4-naphth...
2012-12-01 [Fitoterapia 83(8) , 1336-44, (2012)] |
|
Antipruritic dinaphthofuran-7,12-dione derivatives from the ...
1998-09-01 [J. Nat. Prod. 61(9) , 1126-9, (1998)] |
|
Isolation of an antimicrobial compound from Impatiens balsam...
2001-12-01 [Phytother Res. 15(8) , 676-80, (2001)] |
|
Antifungal activity of lawsone methyl ether in comparison wi...
2011-01-01 [J. Oral. Pathol. Med. 40(1) , 90-6, (2011)] |