4,7-Dichloroquinoline
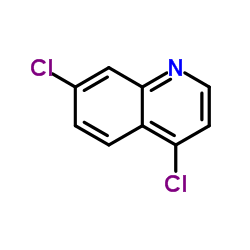
4,7-Dichloroquinoline structure
|
Common Name | 4,7-Dichloroquinoline | ||
|---|---|---|---|---|
| CAS Number | 86-98-6 | Molecular Weight | 198.05 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 292.9±20.0 °C at 760 mmHg | |
| Molecular Formula | C9H5Cl2N | Melting Point | 81-83 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 158.7±7.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
New bioactive compounds from Aloe hijazensis.
Nat. Prod. Res. 23 , 1035-1049, (2009) The chemical constituents and biological activities of leaves and roots of Aloe hijazensis, collected in Saudi Arabia, are reported here for the first time. Twenty-two compounds were obtained, among them eight hydroxyquinones: aloe-emodin (1), emodin (2), chr... |
|
|
Direct, catalytic, and regioselective synthesis of 2-alkyl-, aryl-, and alkenyl-substituted N-heterocycles from N-oxides.
Org. Lett. 16(3) , 864-7, (2014) A one-step transformation of heterocyclic N-oxides to 2-alkyl-, aryl-, and alkenyl-substituted N-heterocycles is described. The success of this broad-scope methodology hinges on the combination of copper catalysis and activation by lithium fluoride or magnesi... |
|
|
"One-pot" synthesis and antimalarial activity of formamidine derivatives of 4-anilinoquinoline.
Chem. Pharm. Bull. 49(8) , 933-7, (2001) Amodiaquine (AQ) is an antimalarial which is effective against chloroquino-resistant strains of Plasmodium falciparum but whose clinical use is severely restricted because of associated hepatotoxicity and agranulocytosis. "One-pot" synthesis of formamidines l... |
|
|
Design, synthesis and potential anti-proliferative activity of some novel 4-aminoquinoline derivatives.
Acta. Pharm. 64(3) , 285-97, (2014) Novel nineteen compounds based on a 4-aminoquinoline scaffold were designed and synthesized as potential antiproliferative agents. The new compounds were N-substituted at the 4-position by aryl or heteroaryl (1-9), quinolin- 3-yl (10), 2-methylquinolin-3-yl (... |
|
|
Allergic contact dermatitis from 4,7-dichloroquinoline.
Contact Dermatitis 8(4) , 269-70, (1982)
|
|
|
Synthesis, cytotoxicity and antileishmanial activity of some N-(2-(indol-3-yl)ethyl)-7-chloroquinolin-4-amines.
Chem. Biol. Drug Des. 75(6) , 628-31, (2010) We report herein the condensation of 4,7-dichloroquinoline (1) with tryptamine (2) and D-tryptophan methyl ester (3). Hydrolysis of the methyl ester adduct (5) yielded the free acid (6). The compounds were evaluated in vitro for activity against four differen... |
|
|
An intermediate for chloroquine analogs: (E)-2-(4,7-dichloro-2-quinolinyl)-3-(dimethylamino)-2-propenal.
Acta Crystallogr. C 51 ( Pt 7) , 1423-5, (1995) The title compound C14H12C12N2O, has been shown to have an E configuration about the double bond in the propenal moiety. Significant delocalization of the lone pair on the N atom of the dimethylamino group into the pi system of this moiety is indicated by the... |
|
|
Identification of an isomer impurity in piperaquine drug substance.
J. Chromatogr. A. 1135(2) , 166-9, (2006) A significant contaminant of the antimalarial drug piperaquine (1,3-bis-[4-(7-chloroquinolyl-4)-piperazinyl-1]propane) has been identified using liquid chromatography-mass spectrometry (LC-MS) and 2D NMR spectroscopy (1H-1H COSY, 1H-13C HSQC, 1H-13C HMBC). Th... |