Chemical Biology & Drug Design
2010-06-01
Synthesis, cytotoxicity and antileishmanial activity of some N-(2-(indol-3-yl)ethyl)-7-chloroquinolin-4-amines.
Elaine S Coimbra, Rafael Carvalhaes, Richard M Grazul, Patricia A Machado, Marcos V N De Souza, Adilson D Da Silva
Index: Chem. Biol. Drug Des. 75(6) , 628-31, (2010)
Full Text: HTML
Abstract
We report herein the condensation of 4,7-dichloroquinoline (1) with tryptamine (2) and D-tryptophan methyl ester (3). Hydrolysis of the methyl ester adduct (5) yielded the free acid (6). The compounds were evaluated in vitro for activity against four different species of Leishmania promastigote forms and for cytotoxic activity against Kb and Vero cells. Compound (5) showed good activity against the Leishmania species tested, while all three compounds displayed moderate activity in both Kb and Vero cells.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
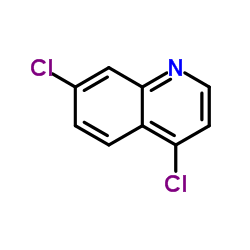 |
4,7-Dichloroquinoline
CAS:86-98-6 |
C9H5Cl2N |
Related Articles:
More...
|
New bioactive compounds from Aloe hijazensis.
2009-01-01 [Nat. Prod. Res. 23 , 1035-1049, (2009)] |
|
Direct, catalytic, and regioselective synthesis of 2-alkyl-,...
2014-02-07 [Org. Lett. 16(3) , 864-7, (2014)] |
|
"One-pot" synthesis and antimalarial activity of formamidine...
2001-08-01 [Chem. Pharm. Bull. 49(8) , 933-7, (2001)] |
|
Design, synthesis and potential anti-proliferative activity ...
2014-09-01 [Acta. Pharm. 64(3) , 285-97, (2014)] |
|
Allergic contact dermatitis from 4,7-dichloroquinoline.
1982-07-01 [Contact Dermatitis 8(4) , 269-70, (1982)] |