| Structure | Name/CAS No. | Articles |
|---|---|---|
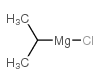 |
ISOPROPYLMAGNESIUM CHLORIDE
CAS:1068-55-9 |
|
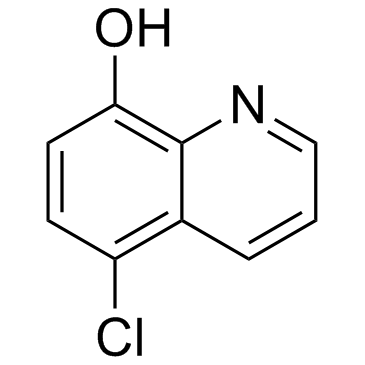 |
5-Chloro-8-hydroxyquinoline
CAS:130-16-5 |
|
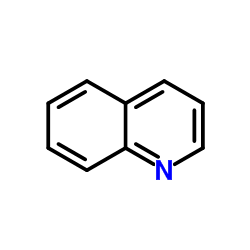 |
leucoline
CAS:91-22-5 |
|
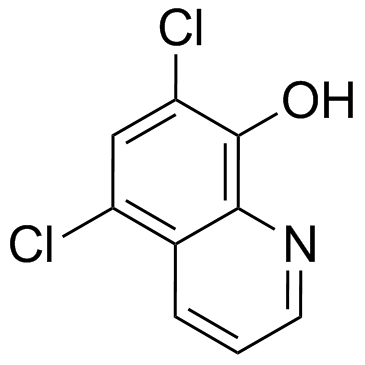 |
Chloroxine
CAS:773-76-2 |
|
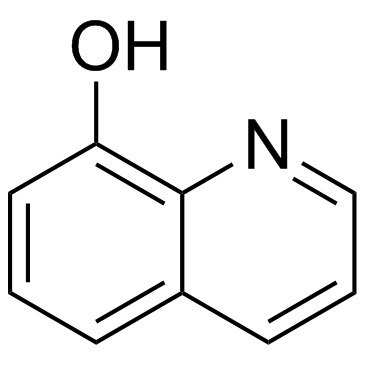 |
8-Hydroxyquinoline
CAS:148-24-3 |
|
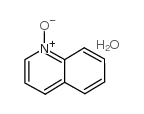 |
quinoline n-oxide hydrate
CAS:64201-64-5 |
|
 |
2-Phenylpyridine
CAS:1008-89-5 |
|
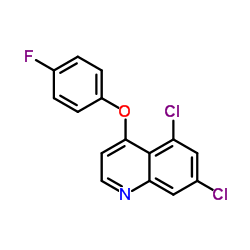 |
Quinoxyfen
CAS:124495-18-7 |
|
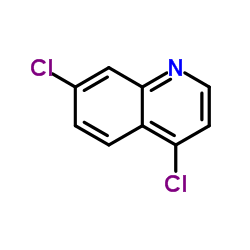 |
4,7-Dichloroquinoline
CAS:86-98-6 |
|
 |
Cinchonidine
CAS:485-71-2 |