COUMERMYCIN AL
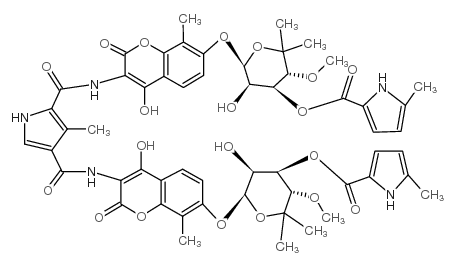
COUMERMYCIN AL structure
|
Common Name | COUMERMYCIN AL | ||
|---|---|---|---|---|
| CAS Number | 4434-05-3 | Molecular Weight | 1110.08000 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C55H59N5O20 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Multiplexing interactions to control antibiotic release from cyclodextrin hydrogels.
Macromol. Biosci. 11(11) , 1544-52, (2011) A new strategy for affinity-based drug delivery by modification of the drug rather than modification of the device is presented. Rifampin is modified to contain either one or two PEG-adamantane arms, and the drug release properties of dimeric coumermycin are ... |
|
|
Aminocoumarins inhibit osteoclast differentiation and bone resorption via downregulation of nuclear factor of activated T cells c1.
Biochem. Pharmacol. 85(3) , 417-25, (2013) Aminocoumarins, such as coumermycin A1 and novobiocin, are natural products of streptomycetes. They are potent inhibitors of bacterial DNA gyrase and are used to suppress the growth of bacteria in inflammatory diseases. However, their effect in osteoclastogen... |
|
|
Modulation of chaperone function and cochaperone interaction by novobiocin in the C-terminal domain of Hsp90: evidence that coumarin antibiotics disrupt Hsp90 dimerization.
J. Biol. Chem. 281 , 7161-7171, (2006) The C-terminal domain of Hsp90 displays independent chaperone activity, mediates dimerization, and contains the MEEVD motif essential for interaction with tetratricopeptide repeat-containing immunophilin cochaperones assembled in mature steroid receptor compl... |
|
|
Synthesis and characterization of PEG-based drug-responsive biohybrid hydrogels.
Macromol. Rapid Commun. 33(15) , 1280-5, (2012) Interactive materials being responsive to a biocompatible stimulus represent a promising approach for future therapeutic applications. In this study, we present a novel biohybrid material synthesized from biocompatible components being stimulus-responsive to ... |
|
|
Covalent CouN7 enzyme intermediate for acyl group shuttling in aminocoumarin biosynthesis.
Chem. Biol. 14(6) , 679-90, (2007) The last stages of assembly of the aminocoumarin antibiotics, clorobiocin and coumermycin A(1), which target the GyrB subunits of bacterial DNA gyrase, involve enzymatic transfer of the pyrrolyl-2-carbonyl acyl group from a carrier protein (CloN1/CouN1) to th... |
|
|
CouO and NovO: C-methyltransferases for tailoring the aminocoumarin scaffold in coumermycin and novobiocin antibiotic biosynthesis.
Biochemistry 44(45) , 14969-76, (2005) During the biosynthesis of the streptomycete aminocoumarin antibiotics novobiocin and the dimeric coumermycin A(1), the bicyclic coumarin scaffold is C-methylated adjacent to the phenolic oxygen. The SAM-dependent C-methyltransferases NovO and CouO have been ... |
|
|
Heterologous expression of the biosynthetic gene clusters of coumermycin A(1), clorobiocin and caprazamycins in genetically modified Streptomyces coelicolor strains.
Biopolymers 93(9) , 823-32, (2010) The biosynthetic gene clusters of the aminocoumarin antibiotics clorobiocin and coumermycin A(1) and of the liponucleoside antibiotic caprazamycin were stably integrated into the genomes of different host strains derived from Streptomyces coelicolor A3(2). Fo... |
|
|
The biosynthetic gene clusters of aminocoumarin antibiotics.
Planta Med. 72(12) , 1093-9, (2006) Plants and microorganisms are the most important sources of secondary metabolites in nature. For research in the functional genomics of secondary metabolism, and for the biotechnological application of such research by genetic engineering and combinatorial bi... |
|
|
Installation of the pyrrolyl-2-carboxyl pharmacophore by CouN1 and CouN7 in the late biosynthetic steps of the aminocoumarin antibiotics clorobiocin and coumermycin A1.
Biochemistry 45(28) , 8568-78, (2006) The 5-methyl-2-pyrrolylcarbonyl moiety of the aminocoumarin antibiotics clorobiocin and coumermycin A1 is the key pharmacophore for targeting the ATP-binding site of GyrB for inhibition of the bacterial type-II topoisomerase DNA gyrase. During the late stage ... |
|
|
Two pathways for pyrrole formation in coumermycin A(1) biosynthesis: the central pyrrole moiety is formed from L-threonine.
ChemBioChem. 12(17) , 2677-85, (2011) Coumermycin A(1) is an aminocoumarin antibiotic produced by Streptomyces rishiriensis. It contains three pyrrole rings, that is, two terminal 5-methyl-pyrrole-2-carboxyl moieties and a central 3-methylpyrrole-2,4-dicarboxylic acid moiety. The biosynthesis of ... |