The biosynthetic gene clusters of aminocoumarin antibiotics.
Shu-Ming Li, Lutz Heide
Index: Planta Med. 72(12) , 1093-9, (2006)
Full Text: HTML
Abstract
Plants and microorganisms are the most important sources of secondary metabolites in nature. For research in the functional genomics of secondary metabolism, and for the biotechnological application of such research by genetic engineering and combinatorial biosynthesis, most microorganisms offer a unique advantage to the researcher: the biosynthetic genes for a specific secondary metabolite are not scattered over the genome, but rather are clustered in a well-defined, contiguous region - the biosynthetic gene cluster of that metabolite. This is exemplified in this review for the biosynthetic gene clusters of the aminocoumarin antibiotics novobiocin, clorobiocin and coumermycin A (1), which are potent inhibitors of DNA gyrase. Cloning, sequencing and analysis of the biosynthetic gene clusters of these three antibiotics revealed that the structural differences and similarities of the compounds are perfectly reflected by the genetic organisation of the biosynthetic gene clusters. The function of most biosynthetic genes could be identified by gene inactivation experiments as well as by heterologous expression and biochemical investigation. The prenylated benzoic acid moiety of novobiocin and clorobiocin, involved in the interaction with gyrase, is structurally similar to metabolites found in plants. However, detailed investigations of the biosynthesis revealed that the biosynthetic pathway and the enzymes involved are totally different from those identified in plants.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
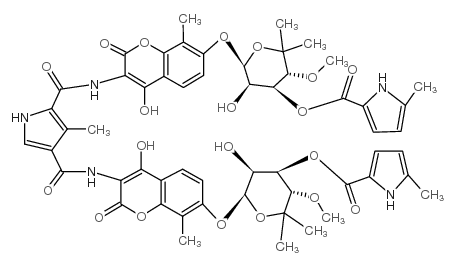 |
COUMERMYCIN AL
CAS:4434-05-3 |
C55H59N5O20 |
|
Multiplexing interactions to control antibiotic release from...
2011-11-10 [Macromol. Biosci. 11(11) , 1544-52, (2011)] |
|
Aminocoumarins inhibit osteoclast differentiation and bone r...
2013-02-01 [Biochem. Pharmacol. 85(3) , 417-25, (2013)] |
|
Modulation of chaperone function and cochaperone interaction...
2006-03-17 [J. Biol. Chem. 281 , 7161-7171, (2006)] |
|
Synthesis and characterization of PEG-based drug-responsive ...
2012-08-14 [Macromol. Rapid Commun. 33(15) , 1280-5, (2012)] |
|
Covalent CouN7 enzyme intermediate for acyl group shuttling ...
2007-06-01 [Chem. Biol. 14(6) , 679-90, (2007)] |