1,3-Cyclohexanedione
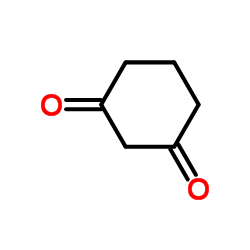
1,3-Cyclohexanedione structure
|
Common Name | 1,3-Cyclohexanedione | ||
|---|---|---|---|---|
| CAS Number | 504-02-9 | Molecular Weight | 112.13 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 235.1±23.0 °C at 760 mmHg | |
| Molecular Formula | C6H8O2 | Melting Point | 101-105 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 85.7±19.6 °C | |
|
Identification and characterization of novel benzil (diphenylethane-1,2-dione) analogues as inhibitors of mammalian carboxylesterases.
J. Med. Chem. 48 , 2906-15, (2005) Carboxylesterases (CE) are ubiquitous enzymes responsible for the metabolism of xenobiotics. Because the structural and amino acid homology among esterases of different classes, the identification of selective inhibitors of these proteins has proved problemat... |
|
|
[Reaction of pyridinium and quinolinium salts having the leaving group at the 2- or 4-position with active methylene compounds].
Yakugaku Zasshi 126(2) , 99-108, (2006) The reactions of 2- or 4-cyanopyridinium salts with active methylene compounds such as dimethyl malonate, malononitrile, and cyclohexane-1,3-dione affording 2- or 4-(substituted methylene) pyridines are described. Similar reactions of 4-cyano-2-methylthiopyri... |
|
|
Dimeric cyclohexane-1,3-dione oximes inhibit wheat acetyl-CoA carboxylase and show anti-malarial activity.
Bioorg. Med. Chem. Lett. 20 , 4611-3, (2010) A series of dimeric 1,3-cyclohexanedione oxime ethers were synthesized and found to have significant antiplasmodial activity with IC(50)'s in the range 3-12 microM. The most active dimer was tested in the Plasmodium berghei mouse model of malaria and at a dos... |
|
|
A structural assignment for a stable acetaldehyde-lysine adduct.
J. Biol. Chem. 270(19) , 11263-6, (1995) Acetaldehyde is the first oxidation product of ethanol in vivo. Lysine residues in proteins such as hemoglobin have been implicated as target structures for acetaldehyde adducts resulting from ethanol consumption. Although the presence of both stable and unst... |
|
|
Chromeno[2,3-d]pyrimidine-triones synthesis by a three-component coupling reaction.
Chem. Pharm. Bull. 58(4) , 516-20, (2010) A simple and one-pot synthesis of new chromeno[2,3-d]pyrimidine-triones by a three-component condensation reaction of barbituric acids, aldehydes and cyclohexane-1,3-diones in refluxing ethanol in the presence of p-toluenesulfonic acid (p-TSA) for 3-10 h is r... |
|
|
Measurement of n-alkanals and hydroxyalkenals in biological samples.
Free Radic. Biol. Med. 15(3) , 281-9, (1993) A modified method was developed to measure nM levels of a range of n-alkanals and hydroxyalkenals in biological samples such as blood plasma and tissue homogenates and also in Folch lipid extracts of these samples. Butylated hydroxytoluene (BHT) and desferrio... |
|
|
Combination of enzyme- and Lewis acid-catalyzed reactions: a new method for the synthesis of 6,7-dihydrobenzofuran-4(5H)-ones starting from 2,5-dimethylfuran and 1,3-cyclohexanediones.
Org. Biomol. Chem. 11(34) , 5692-701, (2013) The Lewis acid-catalyzed domino 1,2-addition/1,4-addition/elimination between (Z)-3-hexene-2,5-dione and 1,3-dicarbonyls delivers 3-methyl-6,7-dihydrobenzofuran-4(5H)-ones exclusively with yields up to 82%. The combination of this new process with the laccase... |
|
|
Improved synthesis of 2,2'-arylmethylene bis(3-hydroxy-5,5-dimethyl-2-cyclohexene-1-one) derivatives catalyzed by urea under ultrasound.
Ultrason. Sonochem. 19(1) , 1-4, (2012) Synthesis of 2,2'-arylmethylene bis(3-hydroxy-5,5-dimethyl-2-cyclohexene-1-one) derivatives catalyzed by urea via the condensation of aromatic aldehydes and 5,5-dimethyl-1,3-cyclohexanedione was carried out in 80-98% yields at 50 °C in aqueous media under ult... |
|
|
Domino alkylation/oxa-Michael of 1,3-cyclohexanediones: steering the C/O-chemoselectivity to reach tetrahydrobenzofuranones.
Org. Biomol. Chem. 9(19) , 6509-12, (2011) An unprecedented domino synthesis of tetrahydrobenzofuran-4-ones is described implicating chemoselective alkylation of various 1,3-cyclohexanediones with bromocrotonate or crotonitrile followed by oxa-Michael cyclization. Further transformations of this core ... |
|
|
EK-2612, a new cyclohexane-1,3-dione possessing selectivity between rice (Oryza sativa) and barnyardgrass (Echinochloa crus-galli).
Pest Manag. Sci. 60(9) , 909-13, (2004) A newly synthesized experimental compound, EK-2612 is one of the class of cyclohexane-1,3-diones which are commonly known to be grasskillers. A greenhouse study was conducted to evaluate the herbicidal performances of EK-2612 on several grass species in compa... |