| Structure | Name/CAS No. | Articles |
|---|---|---|
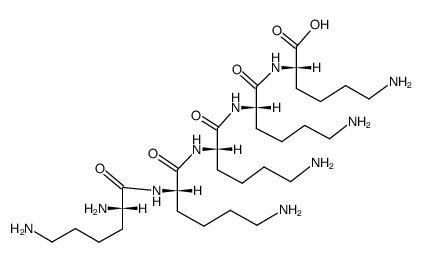 |
H-Lys-Lys-Lys-Lys-Lys-OH acetate salt
CAS:19431-21-1 |
|
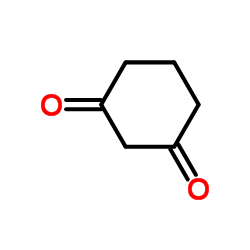 |
1,3-Cyclohexanedione
CAS:504-02-9 |