1,3-Cyclohexanedione
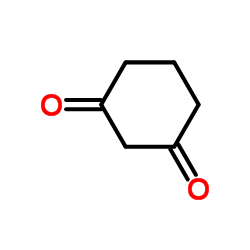
1,3-Cyclohexanedione structure
|
Common Name | 1,3-Cyclohexanedione | ||
|---|---|---|---|---|
| CAS Number | 504-02-9 | Molecular Weight | 112.13 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 235.1±23.0 °C at 760 mmHg | |
| Molecular Formula | C6H8O2 | Melting Point | 101-105 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 85.7±19.6 °C | |
Use of 1,3-CyclohexanedioneCyclohexane-1,3-dione is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | cyclohexane-1,3-dione |
|---|---|
| Synonym | More Synonyms |
| Description | Cyclohexane-1,3-dione is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 235.1±23.0 °C at 760 mmHg |
| Melting Point | 101-105 °C(lit.) |
| Molecular Formula | C6H8O2 |
| Molecular Weight | 112.13 |
| Flash Point | 85.7±19.6 °C |
| Exact Mass | 112.052429 |
| PSA | 34.14000 |
| LogP | -0.89 |
| Vapour Pressure | 0.1±0.5 mmHg at 25°C |
| Index of Refraction | 1.474 |
| InChIKey | HJSLFCCWAKVHIW-UHFFFAOYSA-N |
| SMILES | O=C1CCCC(=O)C1 |
| Storage condition | 2-8°C |
| Water Solubility | soluble |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914299000 |
|---|---|
| Summary | 2914299000. other cyclanic, cyclenic or cyclotherpenic ketones without other oxygen function. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:5.5%. General tariff:30.0% |
|
Identification and characterization of novel benzil (diphenylethane-1,2-dione) analogues as inhibitors of mammalian carboxylesterases.
J. Med. Chem. 48 , 2906-15, (2005) Carboxylesterases (CE) are ubiquitous enzymes responsible for the metabolism of xenobiotics. Because the structural and amino acid homology among esterases of different classes, the identification of ... |
|
|
[Reaction of pyridinium and quinolinium salts having the leaving group at the 2- or 4-position with active methylene compounds].
Yakugaku Zasshi 126(2) , 99-108, (2006) The reactions of 2- or 4-cyanopyridinium salts with active methylene compounds such as dimethyl malonate, malononitrile, and cyclohexane-1,3-dione affording 2- or 4-(substituted methylene) pyridines a... |
|
|
Dimeric cyclohexane-1,3-dione oximes inhibit wheat acetyl-CoA carboxylase and show anti-malarial activity.
Bioorg. Med. Chem. Lett. 20 , 4611-3, (2010) A series of dimeric 1,3-cyclohexanedione oxime ethers were synthesized and found to have significant antiplasmodial activity with IC(50)'s in the range 3-12 microM. The most active dimer was tested in... |
| cyclohexane-1,3-dione |
| Dihydroresorcinol |
| EINECS 207-980-0 |
| 1,3-Cyclohexanedione |
| MFCD00001585 |
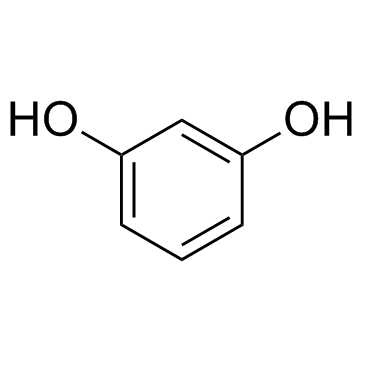 CAS#:108-46-3
CAS#:108-46-3 CAS#:6705-49-3
CAS#:6705-49-3![1,4-Dioxa-spiro[4.5]decan-7-one Structure](https://image.chemsrc.com/caspic/498/4969-01-1.png) CAS#:4969-01-1
CAS#:4969-01-1 CAS#:78743-56-3
CAS#:78743-56-3![7-Oxabicyclo[4.1.0]heptan-2-ol Structure](https://image.chemsrc.com/caspic/053/1192-78-5.png) CAS#:1192-78-5
CAS#:1192-78-5 CAS#:13984-50-4
CAS#:13984-50-4![1,4,8,11-Tetraoxadispiro[4.1.4.3]tetradecane(7CI,8CI,9CI) Structure](https://image.chemsrc.com/caspic/456/177-77-5.png) CAS#:177-77-5
CAS#:177-77-5 CAS#:16807-60-6
CAS#:16807-60-6 CAS#:69016-26-8
CAS#:69016-26-8 CAS#:105501-83-5
CAS#:105501-83-5 CAS#:111596-58-8
CAS#:111596-58-8 CAS#:109704-11-2
CAS#:109704-11-2![2-Cyclohexen-1-one,3-[(2-methyl-2-propenyl)oxy]-(9CI) structure](https://image.chemsrc.com/caspic/131/112148-00-2.png) CAS#:112148-00-2
CAS#:112148-00-2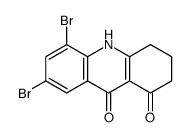 CAS#:106220-41-1
CAS#:106220-41-1![3,4-Dihydro-9-[(benzyl)amino]-1(2H)-acridinone structure](https://image.chemsrc.com/caspic/196/104675-27-6.png) CAS#:104675-27-6
CAS#:104675-27-6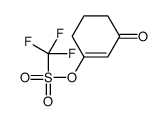 CAS#:109459-28-1
CAS#:109459-28-1 CAS#:104675-23-2
CAS#:104675-23-2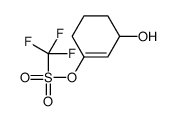 CAS#:109459-30-5
CAS#:109459-30-5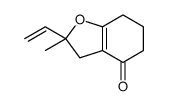 CAS#:109704-10-1
CAS#:109704-10-1
