| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Dimethyl malonate
CAS:108-59-8 |
|
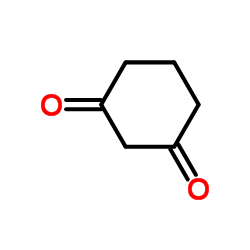 |
1,3-Cyclohexanedione
CAS:504-02-9 |