(+/-)-n-hydroxy-3 4-methylenedioxyamphe&
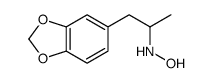
(+/-)-n-hydroxy-3 4-methylenedioxyamphe& structure
|
Common Name | (+/-)-n-hydroxy-3 4-methylenedioxyamphe& | ||
|---|---|---|---|---|
| CAS Number | 114562-59-3 | Molecular Weight | 195.21500 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C10H13NO3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
[Studies on the identification of psychotropic substances. IX. Preparation and various analytical data of reference standard of new psychotropic substances, N-ethyl methylenedioxyamphetamine, N-hydroxy methylenedioxyamphetamine, mecloqualone, 4-methylaminorex, phendimetrazine and phenmetrazine].
Eisei Shikenjo Hokoku. (111) , 66-74, (1993) The reference standards of N-Ethyl methylenedioxyamphetamine, N-Hydroxy methylenedioxy-amphetamine, Mecloqualone, 4-Methylaminorex. Phendimetrazine and Phenmetrazine were chemically prepared from commercial chemicals. Their purities determined by HPLC were mo... |
|
|
In vivo formation of aromatic hydroxylated metabolites of 3,4-(methylenedioxy)methamphetamine in the rat: identification by ion trap tandem mass spectrometric (MS/MS and MS/MS/MS) techniques.
Biol. Mass Spectrom. 20(11) , 677-86, (1991) Aromatic hydroxylation has been established as a pathway for the in vivo metabolism of 3,4-(methylenedioxy)methamphetamine (MDMA) in the rat. Hydroxylation occurred at positions 2, 5 and 6 of the 3,4-methylenedioxyphenyl ring, but is favored at the 6 position... |
|
|
Stimulus effects of N-monoethyl-1-(3,4-methylenedioxyphenyl)-2-aminopropane (MDE) and N-hydroxy-1-(3,4-methylenedioxyphenyl)-2-aminopropane (N-OH MDA) in rats trained to discriminate MDMA from saline.
Pharmacol. Biochem. Behav. 33(4) , 909-12, (1989) Tests of stimulus generalization were conducted using rats trained to discriminate 1.5 mg/kg of N-monomethyl-1-(3,4-methylenedioxyphenyl)-2-aminopropane HCl (MDMA) from saline in order to determine if two structurally related analogs (MDE and N-OH MDA) would ... |
|
|
Determination of a new designer drug, N-hydroxy-3,4-methylenedioxymethamphetamine and its metabolites in rats using ultra-performance liquid chromatography-tandem mass spectrometry.
Forensic Sci. Int. 198(1-3) , 62-9, (2010) An N-hydroxy analogue of 3,4-methylendioxymethamphetamine (MDMA), N-hydroxy MDMA (N-OH MDMA), has recently been distributed as a new designer drug in some drug markets. Very little data is available to the metabolic and pharmacological properties of N-OH MDMA... |
|
|
The pharmacokinetics and liver metabolism of N-hydroxy-3,4-methylenedioxyamphetamine (N-OH MDA) in rats.
Life Sci. 54(26) , PL519-24, (1994) The metabolism and disposition of N-hydroxy-3,4-methylenedioxyamphetamine (N-OH MDA) was studied by utilizing rat liver slices as well as by intravenous pharmacokinetic studies in rats. In the liver slice experiments, N-OH MDA (16 micrograms/ml) was incubated... |
|
|
Liquid chromatographic properties and aqueous solution stability of N-hydroxy-3,4-methylenedioxyamphetamine.
J. Chromatogr. Sci. 28 , 482, (1990) The reversed-phase liquid chromatographic properties of N-hydroxy-3,4-methylenedioxyamphetamine (NOHMDA) were determined on a C8 stationary phase specifically prepared for the separation of basic compounds. NOHMDA and several N-alkyl MDA derivatives displayed... |
|
|
Urinary excretion profiles of N-hydroxy-3,4-methylenedioxymethamphetamine in rats.
Xenobiotica 41(7) , 578-84, (2011) N-hydroxy-3,4-methylenedioxymethamphetamine (N-OH-MDMA) is a psychedelic illicit drug that has recently been circulating in Japan. The aims of this study were (i) to optimise enzymatic hydrolysis conditions of the conjugated forms of N-OH-MDMA and its demethy... |