Determination of a new designer drug, N-hydroxy-3,4-methylenedioxymethamphetamine and its metabolites in rats using ultra-performance liquid chromatography-tandem mass spectrometry.
Ruri Kikura-Hanajiri, Maiko Kawamura, Atsuko Miyajima, Momoko Sunouchi, Yukihiro Goda, Ruri Kikura-Hanajiri, Maiko Kawamura, Atsuko Miyajima, Momoko Sunouchi, Yukihiro Goda, Ruri Kikura-Hanajiri, Maiko Kawamura, Atsuko Miyajima, Momoko Sunouchi, Yukihiro Goda
Index: Forensic Sci. Int. 198(1-3) , 62-9, (2010)
Full Text: HTML
Abstract
An N-hydroxy analogue of 3,4-methylendioxymethamphetamine (MDMA), N-hydroxy MDMA (N-OH MDMA), has recently been distributed as a new designer drug in some drug markets. Very little data is available to the metabolic and pharmacological properties of N-OH MDMA, although it has been reported that the N-demethyl analogue, N-hydroxy-3,4-methylenedioxyamphetamine (N-OH MDA), is mainly metabolized to MDA in rats. In this study, an analytical method for the determination of N-OH MDMA and its metabolites in biological samples was developed, and the metabolic properties of N-OH MDMA in rats were investigated. After the i.p. administration of N-OH MDMA to pigmented hairy rats (5mg/kg/day, 10 days), N-OH MDMA and its N-dehydroxy and N-demethyl metabolites (MDMA, N-OH MDA and MDA) in rat plasma, urine and hair samples were determined by ultra-performance LC (UPLC)-MS/MS. The hair sample was extracted by 1-h sonication and overnight soaking in 5M hydrochloric acid-methanol (1:20). The plasma, urine, and hair extract samples were purified using a solid-phase extraction procedure. N-OH MDMA in the samples could be precisely analyzed by avoiding an alkaline environment. The parent compound very rapidly disappeared from the rat plasma (<15min) and urine (<10h), and most of the N-OH MDMA was excreted in the rat urine as MDMA and MDA in 72h. In the rat hair samples collected 4 weeks after the first administration, N-OH MDMA (0.03ng/mg) and N-OH MDA (0.13ng/mg) were clearly detected as well as MDMA (149ng/mg) and MDA (52ng/mg). This analytical method will be useful for the analysis of N-OH MDMA and its metabolites in biological samples.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
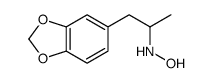 |
(+/-)-n-hydroxy-3 4-methylenedioxyamphe&
CAS:114562-59-3 |
C10H13NO3 |
|
[Studies on the identification of psychotropic substances. I...
1993-01-01 [Eisei Shikenjo Hokoku. (111) , 66-74, (1993)] |
|
In vivo formation of aromatic hydroxylated metabolites of 3,...
1991-11-01 [Biol. Mass Spectrom. 20(11) , 677-86, (1991)] |
|
Stimulus effects of N-monoethyl-1-(3,4-methylenedioxyphenyl)...
1989-08-01 [Pharmacol. Biochem. Behav. 33(4) , 909-12, (1989)] |
|
The pharmacokinetics and liver metabolism of N-hydroxy-3,4-m...
1994-01-01 [Life Sci. 54(26) , PL519-24, (1994)] |
|
Liquid chromatographic properties and aqueous solution stabi...
1990-09-01 [J. Chromatogr. Sci. 28 , 482, (1990)] |