Sakuranetin
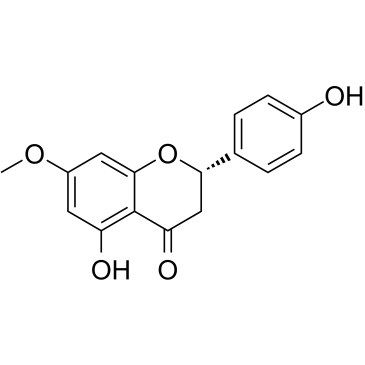
Sakuranetin structure
|
Common Name | Sakuranetin | ||
|---|---|---|---|---|
| CAS Number | 2957-21-3 | Molecular Weight | 286.28 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 555.9±50.0 °C at 760 mmHg | |
| Molecular Formula | C16H14O5 | Melting Point | 153-154ºC | |
| MSDS | Chinese USA | Flash Point | 212.4±23.6 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
A nicotinic receptor-mediated anti-inflammatory effect of the flavonoid rhamnetin in BV2 microglia.
Fitoterapia 98 , 11-21, (2014) The alpha7 nicotinic acetylcholine receptor (nAChR) is a potential target in neuroinflammation. Screening a plant extract library identified Solidago nemoralis as containing methyl-quercetin derivatives that are relatively selective ligands for the alpha7 nAC... |
|
|
In vitro antileishmanial and antitrypanosomal activities of flavanones from Baccharis retusa DC. (Asteraceae).
Exp. Parasitol. 130(2) , 141-5, (2012) Leishmaniasis and Chagas' are parasitic protozoan diseases that affect the poorest population in the world, causing a high mortality and morbidity. As a result of highly toxic and long-term treatments, novel, safe and more efficacious drugs are essential. In ... |
|
|
Isoprenylated flavonoid and adipogenesis-promoting constituents of Dodonaea viscosa.
J. Nat. Prod. 75(4) , 699-706, (2012) Ten new isoprenylated flavonol derivatives, dodoviscins A-J (1-10), and seven known compounds (11-17) were isolated from the aerial parts of Dodonaea viscosa. Compounds 1, 2, 4, 5, 7-9, 5,7,4'-trihydroxy-3',5'-bis(3-methyl-2-buten-1-yl)-3-methoxyflavone (11),... |
|
|
Induced volatiles in elicitor-treated and rice blast fungus-inoculated rice leaves.
Biosci. Biotechnol. Biochem. 66(12) , 2549-59, (2002) The volatiles released from elicitor (copper chloride, jasmonic acid, UV, L-methionine and chitosan oligomer)-treated and rice blast fungus-inoculated rice leaves were collected by the solid-phase microextraction technique and analyzed by GC-MS. (Z)-3-Hexen-1... |
|
|
O-demethylation and sulfation of 7-methoxylated flavanones by Cunninghamella elegans.
Chem. Pharm. Bull. 51(2) , 203-6, (2003) Metabolism of 7-O-methylnaringenin (sakuranetin) by Cunninghamella elegans NRRL 1392 yielded naringenin and naringenin-4'-sulfate. C. elegans also converted 5, 3', 4'-trihydroxy-7-methoxyflavanone into eriodictyol-4'-sulfate. Furthermore, incubation of 5, 4'-... |
|
|
Sakuranetin induces adipogenesis of 3T3-L1 cells through enhanced expression of PPARγ2
Biochem. Biophys. Res. Commun. 372(4) , 835-9, (2008) Sakuranetin (5,4′-dihydroxy-7-methoxyflavone) belongs to the flavanone class of polyphenols predominantly known as phytoalexin in rice plant. In this study, we demonstrate that sakuranetin strongly induces differentiation of 3T3-L1 preadipocytes, as evidenced... |
|
|
Methionine-induced phytoalexin production in rice leaves.
Biosci. Biotechnol. Biochem. 64(3) , 577-83, (2000) The application of methionine on wounded rice leaves induced the production of rice phytoalexins, sakuranetin and momilactone A. This induction resulted from stimulation of phenylalanine ammonia-lyase and naringenin 7-O-methyltransferase activity. Jasmonic ac... |
|
|
Three flavonoids targeting the beta-hydroxyacyl-acyl carrier protein dehydratase from Helicobacter pylori: crystal structure characterization with enzymatic inhibition assay.
Protein Sci. 17(11) , 1971-8, (2008) Flavonoids are the major functional components of many herbal and insect preparations and demonstrate varied pharmacological functions including antibacterial activity. Here by enzymatic assay and crystal structure analysis, we studied the inhibition of three... |
|
|
Stereospecific analysis of sakuranetin by high-performance liquid chromatography: pharmacokinetic and botanical applications.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 875(1) , 136-41, (2008) A stereospecific method for analysis of sakuranetin was developed. Separation was accomplished using a Chiralpak AD-RH column with UV (ultraviolet) detection at 288 nm. The stereospecific linear calibration curves ranged from 0.5 to 100 microg/mL. The mean ex... |
|
|
Evaluation of estrogen receptor Beta binding of pruni cortex and its constituents.
Yakugaku Zasshi 130(7) , 989-97, (2010) The bark of Prunus jamasakura Siebold (Pruni Cortex) has long been used in Japan as a folk remedy and is one of ingredients of the Kampo formula, Jumi-haidoku-to (JHT). JHT is used for treatment of skin diseases such as acne (acne vulgaris). According to Kamp... |