1-amino-2-naphthol hydrochloride
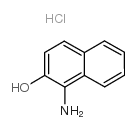
1-amino-2-naphthol hydrochloride structure
|
Common Name | 1-amino-2-naphthol hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 1198-27-2 | Molecular Weight | 195.64500 | |
| Density | N/A | Boiling Point | 336.8ºC at 760mmHg | |
| Molecular Formula | C10H10ClNO | Melting Point | 250 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 157.5ºC | |
| Symbol |


GHS07, GHS08 |
Signal Word | Warning | |
|
Anaerobic metabolism of 1-amino-2-naphthol-based azo dyes (Sudan dyes) by human intestinal microflora.
Appl. Environ. Microbiol. 73(23) , 7759-62, (2007) The rates of metabolism of Sudan I and II and Para Red by human intestinal microflora were high compared to those of Sudan III and IV under anaerobic conditions. Metabolites of the dyes were identified as aniline, 2,4-dimethylaniline, o-toluidine, and 4-nitro... |
|
|
Evaluation of impact of exposure of Sudan azo dyes and their metabolites on human intestinal bacteria.
Anaerobe 18(4) , 445-53, (2012) Sudan azo dyes are banned for food usage in most countries, but they are illegally used to maintain or enhance the color of food products due to low cost, bright staining, and wide availability of the dyes. In this report, we examined the toxic effects of the... |
|
|
Effects of Orange II and Sudan III azo dyes and their metabolites on Staphylococcus aureus.
J. Ind. Microbiol. Biotechnol. 38(10) , 1729-38, (2011) Azo dyes are widely used in the plastic, paper, cosmetics, food, and pharmaceutical industries. Some metabolites of these dyes are potentially genotoxic. The toxic effects of azo dyes and their potential reduction metabolites on Staphylococcus aureus ATCC BAA... |
|
|
Decolorization of azo dyes in bioelectrochemical systems.
Environ. Sci. Technol. 43(13) , 5137-43, (2009) Azo dyes are ubiquitously used in the textile industry. These dyes need to be removed from the effluent prior to discharge to sewage due to their intense color and toxicity. In this study we investigated the use of a bioelectrochemical system (BES) to abiotic... |
|
|
Activation by caecal reduction of the azo dye D & C red no. 9 to a bacterial mutagen.
Mutagenesis 9(4) , 295-9, (1994) D & C Red No. 9 is a monoazo dye used for manufacturing printing inks, rubber and plastics, and as an additive in cosmetics and drugs. In an NTP carcinogenicity study in rats and mice it induced splenic sarcomas and liver nodules in male rats; no chemical-rel... |
|
|
Structural and temperature effects in the high-performance liquid chromatographic enantioseparation of 1-(alpha-aminobenzyl)-2-naphthol and 2-(alpha-aminobenzyl)-1-naphthol analogs.
J. Sep. Sci. 28(18) , 2505-10, (2005) The enantiomers of 1-(alpha-aminobenzyl)-2-naphthol and 2-(alpha-aminobenzyl)-1-naphthol analogs were separated isothermally on a cellulose-tris-3,5-dimethylphenyl carbamate-based chiral stationary phase (Chiralcel OD-H), at 10 degrees C increments in the ran... |
|
|
Anaerobic treatment of azo dye Acid Orange 7 under batch conditions. Méndez-Paz D, et al.
Enzyme Microb. Technol. 36(2) , 264-272, (2005)
|
|
|
Synthesis of photochromic spirooxazines from 1-amino-2-naphthols Lokshin V, et al.
Tetrahedron 53(28) , 9669-9678, (1997)
|