Structural and temperature effects in the high-performance liquid chromatographic enantioseparation of 1-(alpha-aminobenzyl)-2-naphthol and 2-(alpha-aminobenzyl)-1-naphthol analogs.
Anita Sztojkov-Ivanov, István Szatmári, Antal Péter, Ferenc Fülöp
Index: J. Sep. Sci. 28(18) , 2505-10, (2005)
Full Text: HTML
Abstract
The enantiomers of 1-(alpha-aminobenzyl)-2-naphthol and 2-(alpha-aminobenzyl)-1-naphthol analogs were separated isothermally on a cellulose-tris-3,5-dimethylphenyl carbamate-based chiral stationary phase (Chiralcel OD-H), at 10 degrees C increments in the range of 5-35 degrees C, using n-hexane/2-propanol/diethylamine as mobile phase. The mobile phase composition and temperature were varied to achieve baseline resolutions in a single chromatographic run. The dependence of the natural logarithms of selectivity factors, In alpha, on the inverse of temperature, 1/T, was used to determine the thermodynamic data of the enantiomers. The thermodynamic data revealed that all the compounds in this study separate via the same enthalpy-driven chiral recognition mechanism.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
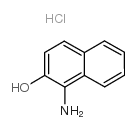 |
1-amino-2-naphthol hydrochloride
CAS:1198-27-2 |
C10H10ClNO |
|
Anaerobic metabolism of 1-amino-2-naphthol-based azo dyes (S...
2007-12-01 [Appl. Environ. Microbiol. 73(23) , 7759-62, (2007)] |
|
Evaluation of impact of exposure of Sudan azo dyes and their...
2012-08-01 [Anaerobe 18(4) , 445-53, (2012)] |
|
Effects of Orange II and Sudan III azo dyes and their metabo...
2011-10-01 [J. Ind. Microbiol. Biotechnol. 38(10) , 1729-38, (2011)] |
|
Decolorization of azo dyes in bioelectrochemical systems.
2009-07-01 [Environ. Sci. Technol. 43(13) , 5137-43, (2009)] |
|
Activation by caecal reduction of the azo dye D & C red no. ...
1994-07-01 [Mutagenesis 9(4) , 295-9, (1994)] |