Phenyl thioacetic acid
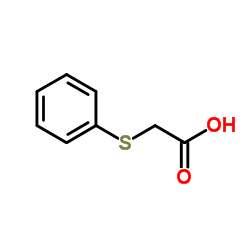
Phenyl thioacetic acid structure
|
Common Name | Phenyl thioacetic acid | ||
|---|---|---|---|---|
| CAS Number | 103-04-8 | Molecular Weight | 168.213 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 314.4±25.0 °C at 760 mmHg | |
| Molecular Formula | C8H8O2S | Melting Point | 60-63 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 144.0±23.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Density functional theory calculations of structure, FT-IR and Raman spectra of S-phenyl thioacetate.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 81(1) , 236-41, (2011) The infrared (4000-400 cm(-1)) and Raman spectra (3700-100 cm(-1)) of liquid S-phenyl thioacetate have been recorded. Molecular geometry, vibrational frequencies and the corresponding assignments were performed by density funtional theory (DFT) using the 6-31... |
|
|
Evaluation of a rapid differentiation test for the Mycobacterium tuberculosis complex by selective inhibition with rho-nitrobenzoic acid and thiophene-2-carboxylic acid hydrazide.
Int. J. Tuberc. Lung Dis. 9(2) , 206-9, (2005) Mycobacterial growth in media to which inhibitory substances are added has been used in species identification. Growth of the Mycobacterium tuberculosis complex (MTC) is inhibited by rho-nitrobenzoic acid (PNB), whereas non-tuberculous mycobacteria (NTM) are ... |
|
|
Identification of two rat liver proteins with paraoxonase activity: biochemical evidence for the identity of paraoxonase and arylesterase.
Chem. Biol. Interact. 119-120 , 263-75, (1999) The existence of two or more enzyme forms with paraoxonase activity has been reported in sheep, rabbit, human and rat serum and recently in mouse and rat liver. In this study we describe the presence of two peaks with paraoxonase activity (M1 and M2) after no... |
|
|
Reactivity of cyclic sulfamidates towards sulfur-stabilised enolates. Stereocontrolled synthesis of functionalised lactams.
Org. Biomol. Chem. 4(10) , 1868-77, (2006) A structurally representative series of 1,2- and 1,3-cyclic sulfamidates react with enolates derived from methyl alpha-phenylthioacetate 9b to give 5- and 6-substituted alpha-phenylthio lactams 20-24. These products provide, via the corresponding sulfoxides, ... |
|
|
Hydrolysis of oxo- and thio-esters by human butyrylcholinesterase.
Biochim. Biophys. Acta 1774(1) , 16-34, (2007) Catalytic parameters of human butyrylcholinesterase (BuChE) for hydrolysis of homologous pairs of oxo-esters and thio-esters were compared. Substrates were positively charged (benzoylcholine versus benzoylthiocholine) and neutral (phenylacetate versus phenylt... |
|
|
Ascorbic acid-dependent turnover and reactivation of 2,4-dichlorophenoxyacetic acid/alpha-ketoglutarate dioxygenase using thiophenoxyacetic acid.
Biochemistry 37(9) , 3035-42, (1998) The first step in catabolism of the broadleaf herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) is catalyzed by 2,4-D/alpha-ketoglutarate (alpha-KG)-dioxygenase (TfdA) in Ralstonia eutropha (formerly Alcaligenes eutrophus) JMP134. This oxygen- and ferrous-ion-... |
|
|
Aerobic microbial degradation of aromatic sulfur-containing compounds and effect of chemical structures.
Chemosphere 36(15) , 3033-41, (1998) Batch data of aerobic microbial degradation rate constants Kb of phenylthio, phenylsulfinyl and phenylsulfonyl acetates have been determined, and the qualitative relationships between their Kb and chemical structures were analyzed. The phenylthio acetates wer... |
|
|
Discovery and SAR of para-alkylthiophenoxyacetic acids as potent and selective PPARdelta agonists.
Bioorg. Med. Chem. Lett. 19(4) , 1101-4, (2009) Synthesis and SAR of para-alkylthiophenoxyacetic acids is described. Achiral compounds 30, 31 and 32 were identified as potent and selective PPARdelta agonists. |
|
|
Discovery of para-alkylthiophenoxyacetic acids as a novel series of potent and selective PPARdelta agonists.
Bioorg. Med. Chem. Lett. 17(14) , 3855-9, (2007) A novel series of potent and selective PPARdelta agonists, para-alkylthiophenoxyacetic acids, was identified. The synthesis and structure-activity relationships are described. |
|
|
Sub-banding and fine structure of serum lactate dehydrogenase isoenzymes induced by sulfur compounds.
Biochem. Biophys. Res. Commun. 101(4) , 1116-22, (1981)
|