Piericidin A1
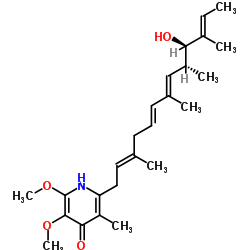
Piericidin A1 structure
|
Common Name | Piericidin A1 | ||
|---|---|---|---|---|
| CAS Number | 2738-64-9 | Molecular Weight | 415.566 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 591.7±50.0 °C at 760 mmHg | |
| Molecular Formula | C25H37NO4 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 311.6±30.1 °C | |
|
Isolation and characterizations of quinone analogue-resistant mutants of bo-type ubiquinol oxidase from Escherichia coli.
Biochemistry 37(37) , 12744-52, (1998) Cytochrome bo is a member of the heme-copper terminal oxidase superfamily and serves as a four-subunit ubiquinol oxidase in the aerobic respiratory chain of Escherichia coli. To probe the location and structural properties of the ubiquinol oxidation site, we ... |
|
|
Etoposide-resistant HT-29 human colon carcinoma cells during glucose deprivation are sensitive to piericidin A, a GRP78 down-regulator.
J. Cell Physiol. 215(1) , 243-50, (2008) Glucose deprivation, a pathophysiological cell condition, causes up-regulation of GRP78 and induction of etoposide resistance in human cancer cells. The induction of drug resistance can be partly explained by the fact that GRP78 can block activation of caspas... |
|
|
Genetic evidence for the existence of two quinone related inhibitor binding sites in NADH-CoQ reductase.
Biochim. Biophys. Acta 1319(1) , 1-4, (1997) Using the NADH-CoQ reductase of Rhodobacter capsulatus as a model for the mitochondrial Complex I, we have for the first time isolated bacterial mutants resistant to piericidin-A, a classical inhibitor of the mitochondrial enzyme. Their sensitivity to other i... |
|
|
Metabolomic identification of the target of the filopodia protrusion inhibitor glucopiericidin A.
Chem. Biol. 17(9) , 989-98, (2010) Identifying the targets of bioactive compounds is a major challenge in chemical biological research. Here, we identified the functional target of the natural bioactive compound glucopiericidin A (GPA) through metabolomic analysis. We isolated GPA while screen... |
|
|
Elucidation of Piericidin A1 biosynthetic locus revealed a thioesterase-dependent mechanism of α-pyridone ring formation.
Chem. Biol. 19(2) , 243-53, (2012) Piericidins are a class of α-pyridone antibiotics that inhibit mitochondrial respiratory chain and exhibit antimicrobial, antifungal, and antitumor activities. Sequential analysis of Streptomyces piomogeues var. Hangzhouwanensis genome revealed six modular po... |
|
|
Molecular characterization of benzimidazole resistance in Helicobacter pylori.
Antimicrob. Agents Chemother. 48(7) , 2524-30, (2004) A family of benzimidazole derivatives (BI) was shown to possess potent and selective activity against Helicobacter pylori, although the precise cellular target of the BIs is unknown. Spontaneous H. pylori mutants were isolated as resistant to a representative... |
|
|
Selective elimination of mitochondria from living cells induced by inhibitors of bioenergetic functions.
Biochem. Soc. Trans. 32(Pt 6) , 1070-1, (2004) The inhibitors of oxidative phosphorylation induced fragmentation of mitochondria without any signs of apoptosis in CV-1 and HeLa cells. Prolonged treatment with the uncouplers (alone or in combination with the inhibitors of respiration) caused perinuclear cl... |
|
|
Inhibition of mitochondrial bioenergetics: the effects on structure of mitochondria in the cell and on apoptosis.
Acta Biochim. Pol. 51(2) , 553-62, (2004) The effects of specific inhibitors of respiratory chain, F(o)F(1)ATP synthase and uncouplers of oxidative phosphorylation on survival of carcinoma HeLa cells and on the structure of mitochondria in the cells were studied. The inhibitors of respiration (pieric... |
|
|
Screening of interleukin-2 production inhibitor with mouse thymoma EL4 cells.
J. Antibiot. 55(11) , 1013-5, (2002)
|
|
|
Enantioselective total syntheses and absolute configuration of JBIR-02 and Mer-A2026B.
Org. Lett. 15(3) , 670-3, (2013) The first total syntheses of the piericidin related natural products Mer-A2026B and JBIR-02 are reported. Key features of the synthetic approach involve an Ir-catalyzed one-pot C-H activation/oxidation procedure for the preparation of the hydroxypyridine, a v... |