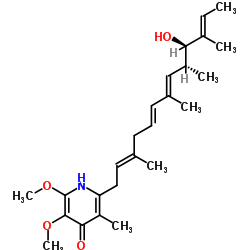
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
YD4588000
-
CHEMICAL NAME :
-
2,6,9,11-Tridecatetraen-4-ol, 13-(4-hydroxy-5,6-dimethoxy-3-methyl-2-pyridyl)-3,5,7 ,11- tetramethyl-, (all-E)-(4S,5S)-
-
CAS REGISTRY NUMBER :
-
2738-64-9
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
5
-
MOLECULAR FORMULA :
-
C25-H37-N-O4
-
MOLECULAR WEIGHT :
-
415.63
-
WISWESSER LINE NOTATION :
-
T6NJ B2UY1&1U2Y1&U1Y1&YQY1&U2 C1 DQ EO1 FO1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
360 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
ABCHA6 Agricultural and Biological Chemistry. (Maruzen Co. Ltd., POB 5050, Tokyo International, Tokyo 100-31, Japan) V.25- 1961- Volume(issue)/page/year: 34,1101,1970
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
3170 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
ABCHA6 Agricultural and Biological Chemistry. (Maruzen Co. Ltd., POB 5050, Tokyo International, Tokyo 100-31, Japan) V.25- 1961- Volume(issue)/page/year: 32,1115,1968
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
2520 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
ABCHA6 Agricultural and Biological Chemistry. (Maruzen Co. Ltd., POB 5050, Tokyo International, Tokyo 100-31, Japan) V.25- 1961- Volume(issue)/page/year: 32,1115,1968
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
870 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
ABCHA6 Agricultural and Biological Chemistry. (Maruzen Co. Ltd., POB 5050, Tokyo International, Tokyo 100-31, Japan) V.25- 1961- Volume(issue)/page/year: 27,576,1963
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JANTAJ Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo, 141, Japan) V.2-5, 1948-52; V.21- 1968- Volume(issue)/page/year: 40,149,1987
|