Piericidin A1
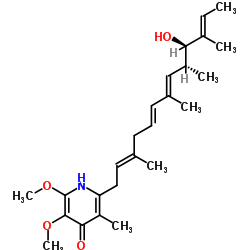
Piericidin A1 structure
|
Common Name | Piericidin A1 | ||
|---|---|---|---|---|
| CAS Number | 2738-64-9 | Molecular Weight | 415.566 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 591.7±50.0 °C at 760 mmHg | |
| Molecular Formula | C25H37NO4 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 311.6±30.1 °C | |
Use of Piericidin A1Piericidin A (AR-054) is a natural mitochondrial NADH-ubiquinone oxidoreductase (complex I) inhibitor. Piericidin A is a potent neurotoxin and inhibits mitochondrial respiration by disrupting the electron transport system through its action on NADH-ubiquinone reductase. Piericidin A is also a potential quorum-sensing inhibitor that suppresses the expression of the virulence genes of Erwinia carotovora subsp. atroseptica (Eca). Piericidin A is an ADC cytotoxin and has anti-bacterial, anticancer, insecticidal activity[1][2][2]. |
| Name | Piericidin A |
|---|---|
| Synonym | More Synonyms |
| Description | Piericidin A (AR-054) is a natural mitochondrial NADH-ubiquinone oxidoreductase (complex I) inhibitor. Piericidin A is a potent neurotoxin and inhibits mitochondrial respiration by disrupting the electron transport system through its action on NADH-ubiquinone reductase. Piericidin A is also a potential quorum-sensing inhibitor that suppresses the expression of the virulence genes of Erwinia carotovora subsp. atroseptica (Eca). Piericidin A is an ADC cytotoxin and has anti-bacterial, anticancer, insecticidal activity[1][2][2]. |
|---|---|
| Related Catalog | |
| In Vitro | In a cell free assay, the potency of Piericidin A to inhibit mitochondrial complex I is ∼2 fold smaller than the one of annonacin. In cultured neurons, Piericidin A potently induces the redistribution of phosphorylated tau from the dendrites into the cell soma and induces cell death[1]. The viability of Tn5B1-4 cells is inhibited by Piericidin A in a time- and concentration-dependent manner with IC50 value of 0.061 μM, whilst Piericidin A shows slight inhibitory effect on the viability of HepG2 and Hek293 cells with IC50 value of 233.97 μM and 228.96 μM, respectively. Piericidin A induces apoptosis of Tn5B1-4 cells coincides with a decrease in the mitochondrial membrane potential[3]. |
| In Vivo | Piericidin A (0.5 mg/kg/d; for 28 days via osmotic minipumps) significantly increases the number of phospho-tau immunoreactive cells in the cerebral cortex in P301S+/+ mice. Piericidin A leads to increased levels of pathologically phosphorylated tau only in P301S+/+ mice. The synaptic density is reduced by Piericidin A treatment in P301S+/+ mice. Exposure to Piericidin A aggravates the course of genetically determined tau pathology[1]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 591.7±50.0 °C at 760 mmHg |
| Molecular Formula | C25H37NO4 |
| Molecular Weight | 415.566 |
| Flash Point | 311.6±30.1 °C |
| Exact Mass | 415.272247 |
| PSA | 71.81000 |
| LogP | 4.26 |
| Vapour Pressure | 0.0±3.8 mmHg at 25°C |
| Index of Refraction | 1.534 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
|---|---|
| Hazard Codes | T+ |
| Risk Phrases | 26/27/28-20/21/22 |
| Safety Phrases | 28-36/37-45 |
| RIDADR | UN 3382 6.1/PG 1 |
|
Isolation and characterizations of quinone analogue-resistant mutants of bo-type ubiquinol oxidase from Escherichia coli.
Biochemistry 37(37) , 12744-52, (1998) Cytochrome bo is a member of the heme-copper terminal oxidase superfamily and serves as a four-subunit ubiquinol oxidase in the aerobic respiratory chain of Escherichia coli. To probe the location and... |
|
|
Etoposide-resistant HT-29 human colon carcinoma cells during glucose deprivation are sensitive to piericidin A, a GRP78 down-regulator.
J. Cell Physiol. 215(1) , 243-50, (2008) Glucose deprivation, a pathophysiological cell condition, causes up-regulation of GRP78 and induction of etoposide resistance in human cancer cells. The induction of drug resistance can be partly expl... |
|
|
Genetic evidence for the existence of two quinone related inhibitor binding sites in NADH-CoQ reductase.
Biochim. Biophys. Acta 1319(1) , 1-4, (1997) Using the NADH-CoQ reductase of Rhodobacter capsulatus as a model for the mitochondrial Complex I, we have for the first time isolated bacterial mutants resistant to piericidin-A, a classical inhibito... |
| Piericidin A1 |
| 2-[(2E,5E,7E,9R,10R,11E)-10-Hydroxy-3,7,9,11-tetramethyl-2,5,7,11-tridecatetraen-1-yl]-5,6-dimethoxy-3-methyl-4(1H)-pyridinone |
| 4(1H)-Pyridinone, 2-[(2E,5E,7E,9R,10R,11E)-10-hydroxy-3,7,9,11-tetramethyl-2,5,7,11-tridecatetraen-1-yl]-5,6-dimethoxy-3-methyl- |
| 2-[(2E,5E,7E,9R,10R,11E)-10-hydroxy-3,7,9,11-tetramethyltrideca-2,5,7,11-tetraen-1-yl]-5,6-dimethoxy-3-methylpyridin-4-ol |
| piericidin a |