Tegafur
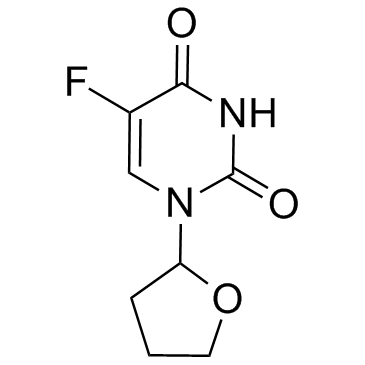
Tegafur structure
|
Common Name | Tegafur | ||
|---|---|---|---|---|
| CAS Number | 17902-23-7 | Molecular Weight | 200.167 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C8H9FN2O3 | Melting Point | 171-173 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS06 |
Signal Word | Danger | |
|
Synergistic antitumor effects of S-1 with eribulin in vitro and in vivo for triple-negative breast cancer cell lines.
Springerplus 3 , 417, (2014) Triple-negative breast cancer (TNBC) is associated with a higher incidence of recurrence and distant metastasis and a poor prognosis, whereas effective treatment strategies remain to be established. Finding an effective treatment for TNBC has become imperativ... |
|
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental drug discovery projects toward safer medicines. In this st... |
|
|
Air to lung partition coefficients for volatile organic compounds and blood to lung partition coefficients for volatile organic compounds and drugs.
Eur. J. Med. Chem. 43 , 478-85, (2008) Values of in vitro gas to lung partition coefficients, K(lung), of VOCs have been collected from the literature. For 44 VOCs, application of the Abraham solvation equation to log K(lung) yielded a correlation with R(2)=0.968 and S.D.=0.25 log units. Combinati... |
|
|
Quantitative structure-activity relationship and complex network approach to monoamine oxidase A and B inhibitors.
J. Med. Chem. 51 , 6740-51, (2008) The work provides a new model for the prediction of the MAO-A and -B inhibitor activity by the use of combined complex networks and QSAR methodologies. On the basis of the obtained model, we prepared and assayed 33 coumarin derivatives, and the theoretical pr... |
|
|
Occurrence and fate of selected anticancer, antimicrobial, and psychotropic pharmaceuticals in an urban river in a subcatchment of the Yodo River basin, Japan.
Environ. Sci. Pollut. Res. Int. 22 , 18676-86, (2015) Pollution status of six anticancer agents in the river water and effluents of sewage treatment plants (STPs) in Japan was surveyed with comparative analysis of the levels of four microbial and one psychotropic pharmaceuticals widely used for therapeutic medic... |
|
|
[An autopsy of G-CSF-producing anaplastic carcinoma of the pancreas with impaired accumulation on FDG-PET after S-1 chemotherapy].
Gan To Kagaku Ryoho. 40(6) , 789-92, (2013) This paper presents a woman in her 70's with G-CSF producing anaplastic carcinoma of the pancreas(Stage IVb)who underwent chemotherapy by S-1 alone. On FDG-PET after the first course, accumulation of FDG was impaired remarkably. After the second course, the p... |
|
|
A phase I/II study of biweekly capecitabine and irinotecan plus bevacizumab as second-line chemotherapy in patients with metastatic colorectal cancer.
Drug Des. Devel. Ther. 9 , 1653-62, (2015) Triweekly capecitabine plus irinotecan (XELIRI) is not completely regarded as a valid substitute for fluorouracil, leucovorin, and irinotecan (FOLFIRI) in metastatic colorectal cancer (mCRC) because of the potential for greater toxicity. We conducted a phase ... |
|
|
News products to avoid.
Prescrire Int. 23(148) , 105, (2014)
|
|
|
Neoadjuvant chemoradiation therapy using concurrent S-1 and irinotecan in rectal cancer: impact on long-term clinical outcomes and prognostic factors.
Int. J. Radiat. Oncol. Biol. Phys. 89(3) , 547-55, (2014) To assess the long-term outcomes of patients with rectal cancer who received neoadjuvant chemoradiation therapy (NCRT) with concurrent S-1 and irinotecan (S-1/irinotecan) therapy.The study group consisted of 115 patients with clinical stage T3 or T4 rectal ca... |
|
|
Celecoxib plus chemoradiotherapy for locally advanced rectal cancer: a phase II TCOG study.
J. Surg. Oncol. 109(6) , 580-5, (2014) To report the results of a phase II trial combining celecoxib and preoperative chemoradiotherapy (CRT) for locally advanced rectal cancer.Patients with clinical stage II or III rectal cancer were treated with radiotherapy of 44 Gy in 22 fractions. Concurrent ... |