SnAP M Reagent
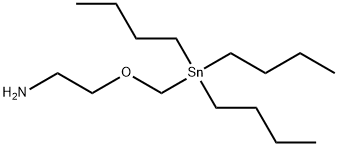
SnAP M Reagent structure
|
Common Name | SnAP M Reagent | ||
|---|---|---|---|---|
| CAS Number | 1557288-04-6 | Molecular Weight | 364.15 | |
| Density | N/A | Boiling Point | 367.9±48.0 °C(Predicted) | |
| Molecular Formula | C15H35NOSn | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |


GHS06, GHS09 |
Signal Word | Danger | |
|
SnAP reagents for the transformation of aldehydes into substituted thiomorpholines--an alternative to cross-coupling with saturated heterocycles.
Angew. Chem. Int. Ed. Engl. 52(6) , 1705-8, (2013)
|
|
|
SnAP reagents for the one-step synthesis of medium-ring saturated N-heterocycles from aldehydes.
Nature Chemistry 6(4) , 310-4, (2014) Interest in saturated N-heterocycles as scaffolds for the synthesis of bioactive molecules is increasing. Reliable and predictable synthetic methods for the preparation of these compounds, especially medium-sized rings, are limited. We describe the developmen... |
|
|
One-step synthesis of saturated spirocyclic N-heterocycles with stannyl amine protocol (SnAP) reagents and ketones.
J. Am. Chem. Soc. 136(51) , 17726-9, (2014) The combination of cyclic ketones and stannyl amine protocol (SnAP) reagents affords saturated, spirocyclic N-heterocycles under operationally simple reaction conditions. The resulting, N-unprotected spirocyclic amines are in great demand as scaffolds for dru... |
|
|
SnAP reagents for the synthesis of piperazines and morpholines.
Org. Lett. 16(4) , 1236-9, (2014) Substituted piperazines and morpholines are valuable structural motifs in biologically active compounds, but are not easily prepared by contemporary cross-coupling approaches. In this report, we introduce SnAP reagents for the transformation of aldehydes into... |