| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
SnAP 2Me-M Reagent
CAS:1557288-07-9 |
|
![3-[(Tributylstannyl)methoxy]-1-propanamine Structure](https://image.chemsrc.com/caspic/112/1577233-70-5.png) |
3-[(Tributylstannyl)methoxy]-1-propanamine
CAS:1577233-70-5 |
|
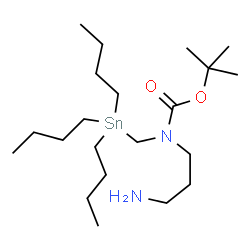 |
SnAP DA Reagent
CAS:1577233-73-8 |
|
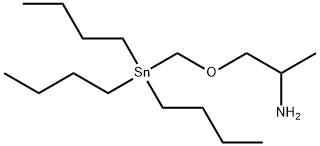 |
SnAP 3Me-M Reagent
CAS:1557288-09-1 |
|
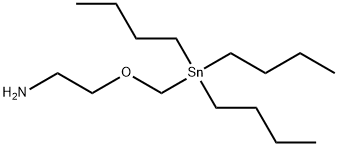 |
SnAP M Reagent
CAS:1557288-04-6 |
|
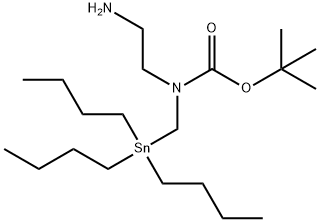 |
SnAP Pip Reagent
CAS:1557287-99-6 |