Vincamine
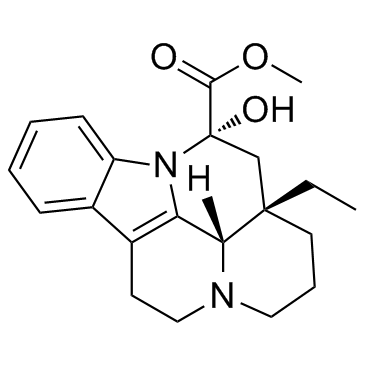
Vincamine structure
|
Common Name | Vincamine | ||
|---|---|---|---|---|
| CAS Number | 1617-90-9 | Molecular Weight | 354.443 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 508.9±50.0 °C at 760 mmHg | |
| Molecular Formula | C21H26N2O3 | Melting Point | 232ºC (dec.) | |
| MSDS | Chinese USA | Flash Point | 261.6±30.1 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Mechanochemical activation of vincamine mediated by linear polymers: assessment of some "critical" steps.
Eur. J. Pharm. Sci. 50(1) , 56-68, (2013) The aim of the research was to investigate three "critical steps" that deserve particular attention during the mechanochemical activation of vincamine. The first step consisted in the selection of the best polymeric carrier/most affine stabiliser between line... |
|
|
Determination of terpenoid indole alkaloids in hairy roots of Rhazya stricta (Apocynaceae) by GC-MS.
Phytochem. Anal. 26 , 331-8, (2015) Rhazya stricta Decne. (Apocynaceae) is a medicinal plant rich in terpenoid indole alkaloids (TIAs), some of which possess important pharmacological properties. The study material including transgenic hairy root cultures have been developed and their potential... |
|
|
Plasma Pharmacokinetics of Polyphenols in a Traditional Japanese Medicine, Jumihaidokuto, Which Suppresses Propionibacterium acnes-Induced Dermatitis in Rats.
Molecules 20 , 18031-46, (2015) Most orally administered polyphenols are metabolized, with very little absorbed as aglycones and/or unchanged forms. Metabolic and pharmacokinetic studies are therefore necessary to understand the pharmacological mechanisms of polyphenols. Jumihaidokuto (JHT)... |
|
|
Smart stability-indicating spectrophotometric methods for determination of binary mixtures without prior separation.
J. AOAC Int. 91(2) , 299-310, (2008) Ratio subtraction and isosbestic point methods are 2 innovating spectrophotometric methods used to determine vincamine in the presence of its acid degradation product and a mixture of cinnarizine (CN) and nicergoline (NIC). Linear correlations were obtained i... |
|
|
Alpneumines A-H, new anti-melanogenic indole alkaloids from Alstonia pneumatophora.
Bioorg. Med. Chem. 18(12) , 4415-21, (2010) Eight new indole alkaloids, alpneumines A-H (1-8) were isolated from the Malaysian Alstonia pneumatophora (Apocynaceae) and their structures were determined by MS and 2D NMR spectroscopic methods. Alpneumines E and G (5 and 7), vincamine, and apovincamine sho... |
|
|
Separation of the four pairs of enantiomers of vincamine alkaloids by enantioselective high-performance liquid chromatography.
J. Chromatogr. A. 893(1) , 47-54, (2000) The four enantiomeric pairs of vincamine group alkaloids were separated by HPLC using Chiralpak AD as chiral stationary phase (CSP) and various n-hexane-2-propanol and n-hexane-ethanol mobile phases. (+)-cis-Vincamine, which is used in pharmaceutical preparat... |
|
|
Measurement and pharmacokinetics of vincamine in rat blood and brain using microdialysis.
J. Chromatogr. A. 1088(1-2) , 146-51, (2005) Vincamine is an alkaloid compound derived from the Vinca minor plant. Since little is known concerning its pharmacokinetics and appropriate analytical method, this study focuses on its pharmacokinetics as well the possible roles of the multidrug transporter P... |
|
|
Stability-indicating methods for determination of vincamine in presence of its degradation product.
J. Pharm. Biomed. Anal. 38(1) , 72-8, (2005) Three different stability indicating assay methods are developed and validated for determination of vincamine in the presence of its degradation product (vincaminic acid). The first method is based on the derivative ratio zero crossing spectrophotometric tech... |
|
|
Singular subsets of locus coeruleus neurons may recover tyrosine hydroxylase phenotype transiently expressed during development.
Brain Res. Mol. Brain Res. 76(2) , 275-81, (2000) The number of tyrosine hydroxylase (TH)-expressing neurons appears to be precisely determined in basal conditions within the noradrenergic pontine nucleus locus coeruleus (LC). However, additional neurons exhibiting TH phenotype have been observed in the adul... |
|
|
Pharmacokinetics and metabolism of vincamine and related compounds.
Eur. J. Drug Metab. Pharmacokinet. 10(2) , 89-103, (1985) The pharmacokinetics and metabolism of vincamine, vinpocetine, methylene-methoxy-apovincaminic acid ester and eburnamine have been reviewed. The main route of elimination for vincamine, vinpocetine and methylene-methoxy-apovincaminic acid ester is ester cleav... |