Journal of Chromatography A
2000-09-29
Separation of the four pairs of enantiomers of vincamine alkaloids by enantioselective high-performance liquid chromatography.
S Caccamese, G Principato
Index: J. Chromatogr. A. 893(1) , 47-54, (2000)
Full Text: HTML
Abstract
The four enantiomeric pairs of vincamine group alkaloids were separated by HPLC using Chiralpak AD as chiral stationary phase (CSP) and various n-hexane-2-propanol and n-hexane-ethanol mobile phases. (+)-cis-Vincamine, which is used in pharmaceutical preparations, is eluted much faster than its optical isomer, with separation factors of 2.4 and 3.5, respectively in these mobile phases. Other CSPs gave negative results. A chiral recognition mechanism is proposed and circular dichroism spectra of the individual enantiomers are presented.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
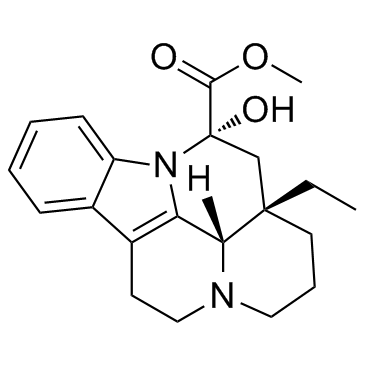 |
Vincamine
CAS:1617-90-9 |
C21H26N2O3 |
Related Articles:
More...
|
Mechanochemical activation of vincamine mediated by linear p...
2013-09-27 [Eur. J. Pharm. Sci. 50(1) , 56-68, (2013)] |
|
Determination of terpenoid indole alkaloids in hairy roots o...
2015-01-01 [Phytochem. Anal. 26 , 331-8, (2015)] |
|
Plasma Pharmacokinetics of Polyphenols in a Traditional Japa...
2015-01-01 [Molecules 20 , 18031-46, (2015)] |
|
Smart stability-indicating spectrophotometric methods for de...
2008-01-01 [J. AOAC Int. 91(2) , 299-310, (2008)] |
|
Alpneumines A-H, new anti-melanogenic indole alkaloids from ...
2010-06-15 [Bioorg. Med. Chem. 18(12) , 4415-21, (2010)] |