fluazifop-P-butyl
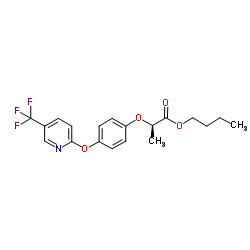
fluazifop-P-butyl structure
|
Common Name | fluazifop-P-butyl | ||
|---|---|---|---|---|
| CAS Number | 79241-46-6 | Molecular Weight | 383.362 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 436.0±45.0 °C at 760 mmHg | |
| Molecular Formula | C19H20F3NO4 | Melting Point | 5ºC | |
| MSDS | Chinese USA | Flash Point | 217.5±28.7 °C | |
| Symbol |



GHS02, GHS08, GHS09 |
Signal Word | Warning | |
|
Effects of low levels of herbicides on prairie species of the Willamette Valley, Oregon.
Environ. Toxicol. Chem. 32(11) , 2542-51, (2013) The relative sensitivity of 17 noncrop plant species from Oregon's Willamette Valley was determined in response to glyphosate, tribenuron methyl (tribenuron), and fluazifop-p-butyl (fluazifop) herbicides. For glyphosate, Elymus trachycaulus, Festuca arundinac... |
|
|
Determination of aryloxyphenoxypropanoic acid derivatives in crops treated with herbicidal sprays.
Z. Lebensm. Unters. Forsch. 190(5) , 436-40, (1990) A reproducible and sensitive gas chromatographic method has been developed for the trace analysis of fluazifop-butyl, haloxyfop-ethoxyethyl and quizalofop-ethyl as well as their main metabolites (corresponding acids analysed after conversion to methyl esters)... |
|
|
Determination of fluazifop-butyl and fluazifop acid in soybeans and soybean oil using liquid chromatography with oxidative amperometric detection.
J. Assoc. Off. Anal. Chem. 72(2) , 368-71, (1989) A new method is described for the determination of the herbicide fluazifop-butyl, and its metabolite fluazifop acid, in soybeans and soybean oil as fluazifop acid. Liquid chromatography with amperometric detection (LC/AD) is used to determine fluazifop acid p... |
|
|
Microbial metabolism of fluazifop-butyl.
J. Environ. Sci. Health B 28(5) , 545-76, (1993) A microbial mixed culture able to grow on fluazifop-butyl and fluazifop was isolated. Fluazifop degradation by this microbial population was studied either when the herbicide was applied as the sole carbon source or in the presence of a second carbon source (... |
|
|
Pharmacokinetics of fluazifop-butyl in human volunteers. II: Dermal dosing.
Hum. Exp. Toxicol. 11(4) , 247-54, (1992) 1. The absorption of the herbicide fluazifop-butyl (f-b), has been determined from plasma and urine measurements in groups of six male volunteers following dermal administration of 2.5, 25 and 250 micrograms cm-2 from standardized formulations containing 0.05... |
|
|
Oral pharmacokinetics of fluazifop-butyl in human volunteers.
Hum. Exp. Toxicol. 10(1) , 39-43, (1991) 1. Fluazifop-butyl, the active ingredient of FUSILADE, a selective herbicide, was administered orally to three male volunteers at a dose level of 0.07 mg kg-1 body weight. Over a period of 6 d between 80 and 93% of the dose was excreted in urine as the metabo... |
|
|
Human xenobiotic metabolizing esterases in liver and blood.
Biochem. Pharmacol. 46(7) , 1125-9, (1993) Esterases in human liver microsomes hydrolysed fluazifop-butyl (Vmax 9.8 +/- 1.6 mumol/min/g tissue), paraoxon (Vmax 47.4 +/- 7.5 nmol/min/g tissue) and phenylacetate (Vmax 57 +/- 8 mumol/min/g tissue), whereas esterases found in the human liver cytosol hydro... |
|
|
Catalytic and structural diversity of the fluazifop-inducible glutathione transferases from Phaseolus vulgaris.
Planta 235(6) , 1253-69, (2012) Plant glutathione transferases (GSTs) comprise a large family of inducible enzymes that play important roles in stress tolerance and herbicide detoxification. Treatment of Phaseolus vulgaris leaves with the aryloxyphenoxypropionic herbicide fluazifop-p-butyl ... |
|
|
Peripheral esterases in the rat: effects of classical inducers.
Chem. Biol. Interact. 87(1-3) , 183-5, (1993) Liver microsomal paraoxonase, aryl esterase and fluazifop butyl esterase (carboxylesterase) were induced by pretreatment of rat with phenobarbitone but not by beta-naphthoflavone or clofibric acid. In the extrahepatic tissues lung cytosolicfluazifop butyl and... |
|
|
A physiologically based mathematical model of dermal absorption in man.
Hum. Exp. Toxicol. 13(1) , 51-60, (1994) A sound understanding of the mechanisms determining percutaneous absorption is necessary for toxicological risk assessment of chemicals contacting the skin. As part of a programme investigating these mechanisms we have developed a physiologically based mathem... |