| Structure | Name/CAS No. | Articles |
|---|---|---|
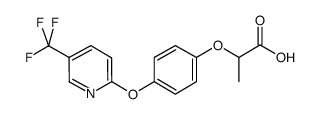 |
fluazifop
CAS:69335-91-7 |
|
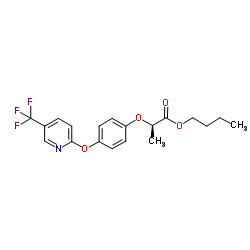 |
fluazifop-P-butyl
CAS:79241-46-6 |
|
![Propanoic acid,2-[4-[[5-(trifluoromethyl)-2-pyridinyl]oxy]phenoxy]-, butyl ester Structure](https://image.chemsrc.com/caspic/312/69806-50-4.png) |
Propanoic acid,2-[4-[[5-(trifluoromethyl)-2-pyridinyl]oxy]phenoxy]-, butyl ester
CAS:69806-50-4 |