4-Fluorobenzeneboronic acid
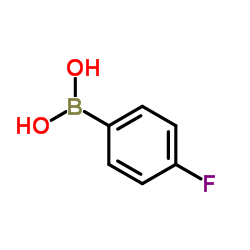
4-Fluorobenzeneboronic acid structure
|
Common Name | 4-Fluorobenzeneboronic acid | ||
|---|---|---|---|---|
| CAS Number | 1765-93-1 | Molecular Weight | 139.92 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 258.4±42.0 °C at 760 mmHg | |
| Molecular Formula | C6H6BFO2 | Melting Point | 262-265 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 110.1±27.9 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Cationic palladium(II)-catalyzed addition of arylboronic acids to nitriles. One-step synthesis of benzofurans from phenoxyacetonitriles.
Org. Lett. 8 , 5987, (2006) [Structure: see text] A cationic palladium complex catalyzed addition of arylboronic acids to nitriles to yield aryl ketones with moderate to good yields was developed. A one-step synthesis of benzofurans from phenoxyacetonitriles under the catalysis of [(bpy... |
|
|
Ruthenium(0)-catalyzed sp3 C-H bond arylation of benzylic amines using arylboronates.
Org. Lett. 7th ed., 14 , 1930-1933, (2012) A Ru-catalyzed direct arylation of benzylic sp(3) carbons of acyclic amines with arylboronates is reported. This highly regioselective and efficient transformation can be performed with various combinations of N-(2-pyridyl) substituted benzylamines and arylbo... |
|
|
Tandem-type Pd(II)-catalyzed oxidative Heck reaction/intramolecular C-H amidation sequence: a novel route to 4-aryl-2-quinolinones.
Chem. Commun. (Camb.) 36th ed., 48 , 4332-4334, (2012) A novel catalytic method for synthesizing 4-aryl-2-quinolinones is reported. The process involves two mechanistically independent, sequential Pd(II)-catalyzed reactions--the oxidative Heck reaction and the intramolecular C-H amidation--both of which smoothly ... |
|
|
Rhodium-catalyzed asymmetric conjugate addition of arylboronic acids to nitroalkenes using olefin-sulfoxide ligands.
J. Org. Chem. 7th ed., 77 , 3071-3081, (2012) An efficient rhodium/olefin-sulfoxide catalyzed asymmetric conjugate addition of organoboronic acids to a variety of nitroalkenes has been developed, where 2-methoxy-1-naphthyl sulfinyl functionalized olefin ligands have shown to be highly effective and are a... |
|
|
Tetrahedron Lett. 48 , 845, (2007)
|
|
|
A copper-catalyzed Petasis reaction for the synthesis of tertiary amines and amino esters.
J. Org. Chem. 9th ed., 77 , 4445-4449, (2012) We have developed a copper-catalyzed process for the coupling of aldehydes, amines, and boronic acids. This allows greater reactivity with simple aryl boronic acids and allows coupling reactions to proceed that previously failed. Initial mechanistic studies s... |
|
|
Palladium-Catalyzed Cross-Coupling of Organoboron Compounds with Iodonium Salts and Iodanes.
J. Org. Chem. 61 , 4720, (1996) The palladium-catalyzed cross-coupling reaction of iodinanes (iodonium salts and iodanes) with organoboron compounds to form carbon-carbon bonds was achieved at ambient temperature under aqueous conditions in the absence of base. Coupling of phenylboronic aci... |
|
|
Tetrahedron Lett. 37 , 3857, (1996)
|
|
|
Microwave-enhanced triton B catalyzed Suzuki coupling reaction Meshram, H. M.; et al.
Indian J. Chem. B 2nd ed., 51 , 362-365, (2012)
|
|
|
Pd-catalyzed direct arylation of phenylpyrazole: Synthesis of fipronil derivatives with aryl boronic acids promoted by a stoichiometric amount of NIS Zhang, X-H.; Han, J-S.; Zhong, P.
J. Fluor. Chem. 137 , 44-49, (2012)
|