| Structure | Name/CAS No. | Articles |
|---|---|---|
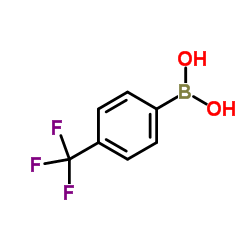 |
4-Trifluoromethylphenylboronic acid
CAS:128796-39-4 |
|
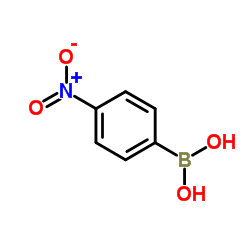 |
(4-Nitrophenyl)boronic acid
CAS:24067-17-2 |
|
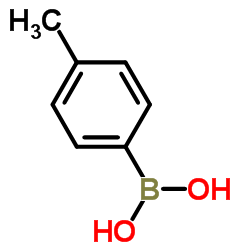 |
4-Tolylboronic acid
CAS:5720-05-8 |
|
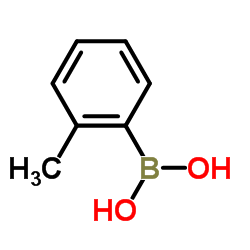 |
tolylboronic acid
CAS:16419-60-6 |
|
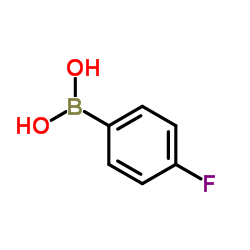 |
4-Fluorobenzeneboronic acid
CAS:1765-93-1 |
|
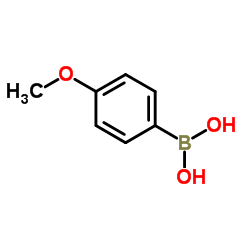 |
4-Methoxyphenylboronic acid
CAS:5720-07-0 |
|
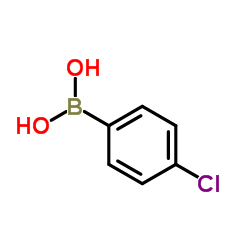 |
(4-Chlorophenyl)boronic acid
CAS:1679-18-1 |
|
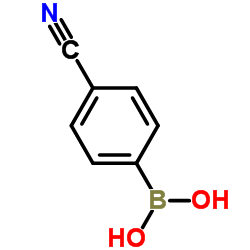 |
4-Cyanophenylboronic acid
CAS:126747-14-6 |