4-Tolylboronic acid
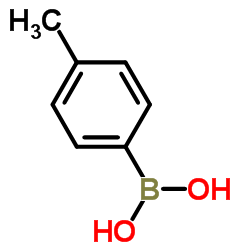
4-Tolylboronic acid structure
|
Common Name | 4-Tolylboronic acid | ||
|---|---|---|---|---|
| CAS Number | 5720-05-8 | Molecular Weight | 135.956 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 275.2±33.0 °C at 760 mmHg | |
| Molecular Formula | C7H9BO2 | Melting Point | 256-263 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 120.2±25.4 °C | |
|
Carbonic anhydrase inhibitors. Inhibition of the fungal beta-carbonic anhydrases from Candida albicans and Cryptococcus neoformans with boronic acids.
Bioorg. Med. Chem. Lett. 19 , 2642-5, (2009) Inhibition of the beta-carbonic anhydrases (CAs, EC 4.2.1.1) from the pathogenic fungi Cryptococcus neoformans (Can2) and Candida albicans (Nce103) with a series of aromatic, arylalkenyl- and arylalkylboronic acids was investigated. Aromatic, 4-phenylsubstitu... |
|
|
Direct palladium(II)-catalyzed synthesis of arylamidines from aryltrifluoroborates.
Org. Lett. 9th ed., 14 , 2394-2397, (2012) A fast and convenient synthesis of arylamidines starting from readily available potassium aryltrifluoroborates and cyanamides is reported. The coupling was achieved by Pd(II)-catalysis in a one step 20 min microwave protocol using Pd(O(2)CCF(3)), 6-methyl-2,2... |
|
|
Ruthenium(0)-catalyzed sp3 C-H bond arylation of benzylic amines using arylboronates.
Org. Lett. 7th ed., 14 , 1930-1933, (2012) A Ru-catalyzed direct arylation of benzylic sp(3) carbons of acyclic amines with arylboronates is reported. This highly regioselective and efficient transformation can be performed with various combinations of N-(2-pyridyl) substituted benzylamines and arylbo... |
|
|
Tandem-type Pd(II)-catalyzed oxidative Heck reaction/intramolecular C-H amidation sequence: a novel route to 4-aryl-2-quinolinones.
Chem. Commun. (Camb.) 36th ed., 48 , 4332-4334, (2012) A novel catalytic method for synthesizing 4-aryl-2-quinolinones is reported. The process involves two mechanistically independent, sequential Pd(II)-catalyzed reactions--the oxidative Heck reaction and the intramolecular C-H amidation--both of which smoothly ... |
|
|
Aryl boronic acid inhibition of synthetic melanin polymerization.
Bioorg. Med. Chem. Lett. 20 , 4475-8, (2010) Inhibitors of melanin formation are sought after for a range of applications. Boronophenylalanine is known to inhibit melanogenesis via boronic acid-catechol interactions. A spectroscopic assay was developed to study the polymerization of l-dopa to synthetic ... |
|
|
Rhodium-catalyzed asymmetric conjugate addition of arylboronic acids to nitroalkenes using olefin-sulfoxide ligands.
J. Org. Chem. 7th ed., 77 , 3071-3081, (2012) An efficient rhodium/olefin-sulfoxide catalyzed asymmetric conjugate addition of organoboronic acids to a variety of nitroalkenes has been developed, where 2-methoxy-1-naphthyl sulfinyl functionalized olefin ligands have shown to be highly effective and are a... |
|
|
Design and synthesis of boronic acid inhibitors of endothelial lipase.
Bioorg. Med. Chem. Lett. 22 , 1397-401, (2012) Endothelial lipase (EL) and lipoprotein lipase (LPL) are homologous lipases that act on plasma lipoproteins. EL is predominantly a phospholipase and appears to be a key regulator of plasma HDL-C. LPL is mainly a triglyceride lipase regulating (V)LDL levels. T... |
|
|
Pd-catalyzed direct arylation of phenylpyrazole: Synthesis of fipronil derivatives with aryl boronic acids promoted by a stoichiometric amount of NIS Zhang, X-H.; Han, J-S.; Zhong, P.
J. Fluor. Chem. 137 , 44-49, (2012)
|
|
|
Ligand-free copper-catalyzed coupling of nitroarenes with arylboronic acids Zhang, J.; et al.
Green Chem. 4th ed., 14 , 912-916, (2012)
|
|
|
Regioselective Arylation and Alkynylation of 2,3-Dibromo-1H-inden-1-one by Suzuki-Miyaura and Sonogashira Cross-Coupling Reactions Khera, R. A.; et al.
Helv. Chim. Acta 3rd ed., 95 , 469-482, (2012)
|