Palladium-Catalyzed Cross-Coupling of Organoboron Compounds with Iodonium Salts and Iodanes.
Suk-Ku Kang, Hong-Woo Lee, Su-Bum Jang, Pil-Su Ho
Index: J. Org. Chem. 61 , 4720, (1996)
Full Text: HTML
Abstract
The palladium-catalyzed cross-coupling reaction of iodinanes (iodonium salts and iodanes) with organoboron compounds to form carbon-carbon bonds was achieved at ambient temperature under aqueous conditions in the absence of base. Coupling of phenylboronic acid with diphenyliodonium tetrafluoroborate in the presence of Pd(PPh(3))(4) (0.2 mol %) or Pd(OAc)(2) (0.2 mol %) under aqueous conditions gave biphenyl in almost quantitative yield. Under the same conditions, substituted boronic acids, boronates, and trialkylboranes were readily coupled with diaryl-, alkenyl-, and alkynyliodonium salts. Finally, the iodanes ArI(OH)OTs underwent cross-coupling with boronic acids, boronates, and trialkylboranes to afford biphenyls and aryl-substituted alkenes.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
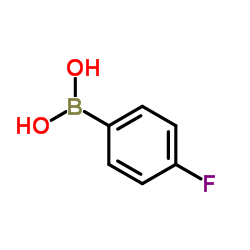 |
4-Fluorobenzeneboronic acid
CAS:1765-93-1 |
C6H6BFO2 |
|
Cationic palladium(II)-catalyzed addition of arylboronic aci...
2006-12-21 [Org. Lett. 8 , 5987, (2006)] |
|
Ruthenium(0)-catalyzed sp3 C-H bond arylation of benzylic am...
2012-04-06 [Org. Lett. 7th ed., 14 , 1930-1933, (2012)] |
|
Tandem-type Pd(II)-catalyzed oxidative Heck reaction/intramo...
2012-05-07 [Chem. Commun. (Camb.) 36th ed., 48 , 4332-4334, (2012)] |
|
Rhodium-catalyzed asymmetric conjugate addition of arylboron...
2012-04-06 [J. Org. Chem. 7th ed., 77 , 3071-3081, (2012)] |
|
[Tetrahedron Lett. 48 , 845, (2007)] |