对乙酰氨基苯酚
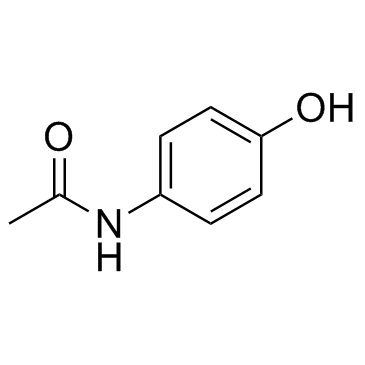
对乙酰氨基苯酚结构式

|
常用名 | 对乙酰氨基苯酚 | 英文名 | 4-Acetamidophenol |
|---|---|---|---|---|
| CAS号 | 103-90-2 | 分子量 | 151.163 | |
| 密度 | 1.3±0.1 g/cm3 | 沸点 | 387.8±25.0 °C at 760 mmHg | |
| 分子式 | C8H9NO2 | 熔点 | 168-172 °C(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 188.4±23.2 °C | |
| 符号 |

GHS07 |
信号词 | Warning |
对乙酰氨基苯酚用途Acetaminophen (paracetamol) 是选择性环氧合酶-2 (COX-2) 的抑制剂,IC50 值为 25.8 μM。它是广泛使用的解热和止痛药。 |
||||
对乙酰氨基苯酚作用本品为解热镇痛药,通过抑制环氧化酶,选择性抑制下丘脑体温调节中枢前列腺素的合成,导致外周血管扩张、出汗而达到解热的作用,其解热作用强度与阿司匹林相似;通过抑制前列腺素等的合成和释放,提高痛阈而起到镇痛作用,属于外周性镇痛药,作用较阿司匹林弱,仅对轻、中度疼痛有效。本品无明显抗炎作用。 |
| 中文名 | N-乙酰对氨基酚 |
|---|---|
| 英文名 | paracetamol |
| 中文别名 | 4-乙酰胺基苯酚 | 4'-羟基乙酰苯胺 | 对羟基乙酰苯胺 | 4-乙酰氨基酚 | 对乙酰氨基苯酚 | 对乙酰氨基酚 | 扑热息痛 | N-(4-羟基苯基)乙酰胺 |
| 英文别名 | 更多 |
| 描述 | Acetaminophen (paracetamol) 是选择性环氧合酶-2 (COX-2) 的抑制剂,IC50 值为 25.8 μM。它是广泛使用的解热和止痛药。 |
|---|---|
| 相关类别 | |
| 靶点实验 |
COX-2:25.8 μM (IC50) COX-1:113.7 μM (IC50) Human Endogenous Metabolite |
| 体外研究 | 在体外,对乙酰氨基酚引起对COX-2抑制的4.4倍选择性(COX-1的IC50为113.7μM; COX-2的IC50为25.8μM)。口服给药后,最大离体抑制为56%(COX-1)和83%(COX-2)。在给药后至少5小时,对乙酰氨基酚血浆浓度保持在COX-2的体外IC 50之上。离体IC 50值(COX-1:105.2μM; COX-2:26.3μM)的对乙酰氨基酚与其体外IC 50值相比是有利的。与先前的概念相反,对乙酰氨基酚抑制COX-2超过80%,即与非甾体抗炎药(NSAID)和选择性COX-2抑制剂相当的程度。然而,没有达到> 95%COX-1阻断与抑制血小板功能有关[1]。 MTT测定显示,50mM剂量的对乙酰氨基酚(APAP)显着(p <0.001)使细胞活力降低至61.5±6.65%。有趣的是,与对乙酰氨基酚处理的细胞相比,在对乙酰氨基酚/ HV110共处理细胞中观察到细胞存活率显着(p <0.01)增加至79.7±2.47%[2]。 |
| 体内研究 | 对小鼠施用对乙酰氨基酚(250 mg/kg,口服)导致肝脏损伤和细胞坏死显着(p <0.001),血清肝酶丙氨酸氨基转移酶(ALT),氨基转移酶(AST),碱性磷酸酶(ALP)升高证明和γ-谷氨酰转移酶(γGT)与正常组比较。相反,不同剂量柠檬醛(125,250和500 mg/kg)预处理的效果显示ALT血清活性显着(p <0.05)降低(分别为91.79%,93.07%和95.61%), AST(分别为93.40%,91.89%和96.52%),ALP(分别为39.29%,37.07%和59.80%)和γGT(分别为92.83%,91.59%和93.0%),与对乙酰氨基酚组相比。 SLM预处理对ALT活性(95.90%),AST(95.03%),ALP(70.52%)和γGT(92.69%)的影响相似[3]。 |
| 细胞实验 | 人肝癌细胞系HepG2在补充有10%胎牛血清(FBS),100U / mL青霉素和100μg/ mL链霉素和2mM L-谷氨酰胺的低葡萄糖DMEM中培养。将细胞保持在37cm的75cm 2烧瓶中,在含有5%CO 2的潮湿气氛中,并且每5天以80%汇合度分开。将细胞接种在24孔板(2×10 5个细胞)中,并在37℃下孵育过夜,然后用含有高葡萄糖浓度的完全DMEM预处理细胞,以下调自噬。 6小时后,用从发酵乳杆菌BGHV110菌株(HV110)获得的不同浓度的后生物处理细胞,以选择合适的剂量用于进一步的实验。将生物素溶解在完全DMEM培养基中并以特定的最终浓度添加到细胞中。在所有其他实验中,接种细胞用50mM对乙酰氨基酚单独处理或用50mM对乙酰氨基酚和选定剂量的冻干HV110共处理。为了分析自噬通量,在处理的同时,将细胞暴露于浓度为25μM的溶酶体药物氯喹,以抑制自噬体 - 溶酶体融合[2]。 |
| 动物实验 | 小鼠[3]使用雄性Swiss小鼠(30-40g)。将实验动物分成六组,每组五只动物。首先,每组在7天内口服接受以下治疗:第I组:小鼠未接受任何治疗(正常)。第II组:小鼠接受柠檬醛载体(0.1%吐温80溶液)。组III-V:小鼠分别以125,250和500mg / kg的剂量用柠檬醛预处理。第VI组:用保肝标准药物水飞蓟素(SLM)(200mg / kg)预处理小鼠。此后,动物禁食8小时,然后在第7天以250mg / kg的剂量在组II-VI中接受口服对乙酰氨基酚。组I口服接受含有0.1%吐温80溶液(对乙酰氨基酚载体)的盐水。将储备溶液用作50mg / mL的第一浓度,然后在0.1%吐温80溶液中稀释以制备25和12.5mg / mL的溶液。给予对乙酰氨基酚12小时后,收集血清样品和肝组织,然后进行生物化学和组织学分析。 |
| 参考文献 |
| 密度 | 1.3±0.1 g/cm3 |
|---|---|
| 沸点 | 387.8±25.0 °C at 760 mmHg |
| 熔点 | 168-172 °C(lit.) |
| 分子式 | C8H9NO2 |
| 分子量 | 151.163 |
| 闪点 | 188.4±23.2 °C |
| 精确质量 | 151.063324 |
| PSA | 49.33000 |
| LogP | 0.34 |
| InChIKey | RZVAJINKPMORJF-UHFFFAOYSA-N |
| SMILES | CC(=O)Nc1ccc(O)cc1 |
| 外观性状 | 白色粉末或晶体 |
| 蒸汽压 | 0.0±0.9 mmHg at 25°C |
| 折射率 | 1.619 |
| 储存条件 | 1.储存于阴凉、通风的库房。远离火种、热源。保持容器密封。应与氧化剂分开存放,切忌混储。配备相应品种和数量的消防器材。储区应备有合适的材料收容泄漏物。 |
| 稳定性 | 1.避免与氧化物接触。 2.有毒,具有刺激性,使用时应避免与眼睛及皮肤接触。 |
| 水溶解性 | 14 g/L (20 ºC) |
| 分子结构 | 1、 摩尔折射率:42.40 2、 摩尔体积(cm3/mol):120.9 3、 等张比容(90.2K):326.0 4、 表面张力(dyne/cm):52.8 5、 介电常数: 6、 偶极距(10-24cm3): 7、 极化率:16.81 |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:2 3.氢键受体数量:2 4.可旋转化学键数量:1 5.互变异构体数量:6 6.拓扑分子极性表面积49.3 7.重原子数量:11 8.表面电荷:0 9.复杂度:139 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
| 更多 | 1. 性状:无色单斜方棱形式结晶。无臭。味苦。 2. 密度(g/mL,25/4℃): 1.293 3. 相对蒸汽密度(g/mL,空气=1):未确定 4. 熔点(ºC):169-170 5. 沸点(ºC,常压):未确定 6. 沸点(ºC,5.2kPa):未确定 7. 折射率:未确定 8. 闪点(ºC):未确定 9. 比旋光度(º):未确定 10. 自燃点或引燃温度(ºC):未确定 11. 蒸气压(kPa,25ºC):未确定 12. 饱和蒸气压(kPa,60ºC):未确定 13. 燃烧热(KJ/mol):未确定 14. 临界温度(ºC):未确定 15. 临界压力(KPa):未确定 16. 油水(辛醇/水)分配系数的对数值:未确定 17. 爆炸上限(%,V/V):未确定 18. 爆炸下限(%,V/V):未确定 19.溶解性:溶于甲醇、乙醇、二氯乙烯、丙酮和乙酸乙酯,微溶于乙醚和热水,几乎不溶于冷水,不溶于石油醚、戊烷和苯。 |
|
4'-羟基乙酰苯胺
修改号码:6
模块1. 化学品 产品名称: 4'-Hydroxyacetanilide 修改号码: 6 模块2. 危险性概述 GHS分类 物理性危害未分类
健康危害 急性毒性(经口) 第4级 生殖细胞敏感性 第2级 特异性靶器官毒性 肝脏, 消化道, 心脏, 肾脏, 中枢神经系统 - 单一接触 [第1级] 特异性靶器官毒性 呼吸系统, 睾丸 - 单一接触 [第2级] 特异性靶器官毒性 肝脏, 血液(系统), 肾脏 - 单一接触 [第1级] 特异性靶器官毒性甲状腺 - 单一接触 [第2级] 环境危害 急性水生毒性 第2级 慢性水生毒性 第2级 GHS标签元素 图标或危害标志 信号词危险 危险描述吞咽有害。 怀疑会造成遗传缺陷 对器官引起损害: 肝脏 消化道 心脏 肾脏 中枢神经系统 可能对器官产生损害: 呼吸系统 睾丸 可能因延长或接触对器官产生损害: 肝脏 血液(系统) 肾 脏 可能因延长或接触对器官产生损害: 甲状腺 4'-羟基乙酰苯胺 修改号码:6 模块2. 危险性概述 对水生生物有毒性 长期影响对水生生物有毒性 防范说明 [预防]使用前获取特定手册。 处理前必须阅读并理解所有安全措施。 切勿吸入。 避免释放到环境中。 使用本产品时切勿吃东西,喝水或吸烟。 处理后要彻底清洗双手。 使用个人所需的防护用具。 [急救措施] 食入:若感不适,呼叫解毒中心/医生。漱口。 如接触到或相关接触:求医/就诊。 收集溢出物。 [储存]存放处须加锁。 [废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。 模块3. 成分/组成信息 单一物质/混和物单一物质 化学名(中文名): 4'-羟基乙酰苯胺 百分比: >98.0%(HPLC)(N) CAS编码: 103-90-2 俗名: 4-Acetamidophenol , Acetaminophen , Paracetamol 分子式: C8H9NO2 模块4. 急救措施 吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。求医/就诊。 皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。 求医/就诊。 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。 求医/就诊。 食入: 求医/就诊。漱口。 危害迹象: 腹痛, 腹泻, 嗜睡, 恶心, 意识丧失, 呕吐 紧急救助者的防护:救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。 医生注意事项:建议医学观察。 模块5. 消防措施 合适的灭火剂:干粉,泡沫,雾状水,二氧化碳 特殊危险性:小心,燃烧或高温下可能分解产生毒烟。 特定方法:从上风处灭火,根据周围环境选择合适的灭火方法。 非相关人员应该撤离至安全地方。 周围一旦着火:如果安全,移去可移动容器。 消防员的特殊防护用具:灭火时,一定要穿戴个人防护用品。 模块6. 泄漏应急处理 个人防护措施,防护用具, 使用特殊的个人防护用品(针对有毒颗粒的P3过滤式空气呼吸器)。远离溢出物/泄露 紧急措施:处并处在上风处。 泄露区应该用安全带等圈起来,控制非相关人员进入。 环保措施:小心,切勿排入河流等。因为考虑对环境有负面影响。 4'-羟基乙酰苯胺 修改号码:6 模块6. 泄漏应急处理 控制和清洗的方法和材料:清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的 法律法规处置。 模块7. 操作处置与储存 处理 技术措施:在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手 和脸。 注意事项:如果可能,使用封闭系统。如果粉尘或浮质产生,使用局部排气。 操作处置注意事项:避免所有部位的接触! 贮存 储存条件:保持容器密闭。存放于凉爽、阴暗处。 存放处须加锁。 远离不相容的材料比如氧化剂存放。 包装材料:依据法律。 模块8. 接触控制和个体防护 工程控制:尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。 个人防护用品 呼吸系统防护: 防尘面具,自携式呼吸器(SCBA),供气呼吸器等。使用通过政府标准的呼吸器。依 据当地和政府法规。 手部防护:防渗手套。 眼睛防护:护目镜。如果情况需要,佩戴面具。 皮肤和身体防护:防渗防护服。如果情况需要,穿戴防护靴。 模块9. 理化特性 固体 外形(20°C): 外观: 晶体-粉末 颜色:白色类白色 气味:无味 pH: 5.5 - 6.5 (saturated soln.H2O) 熔点: 169°C 沸点/沸程无资料 闪点:无资料 爆炸特性 爆炸下限:无资料 爆炸上限:无资料 蒸气压: 9.3x10-4Pa/25°C 密度:无资料 溶解度: [水] 微溶于(1.4g/100mL, 20°C) [其他溶剂] 溶于: 丙酮, 乙醇, 二甲基甲酰胺, 乙酸乙酯 微溶于:醚 不溶于: 苯, 石油醚 log水分配系数 = 0.49 模块10. 稳定性和反应性 化学稳定性:一般情况下稳定。 危险反应的可能性:未报道特殊反应性。 4'-羟基乙酰苯胺 修改号码:6 模块10. 稳定性和反应性 须避免接触的物质氧化剂 危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx) 模块11. 毒理学信息 急性毒性: orl-rat LD50:1944 mg/kg scu-mus LD50:310 mg/kg ipr-rat LD50:1205 mg/kg orl-hmn LDLo:143 mg/kg 对皮肤腐蚀或刺激:无资料 对眼睛严重损害或刺激:无资料 生殖细胞变异原性: cyt-hmn-lym 200 mg/L cyt-hmn-orl 42860 ug/kg dns-rat-orl 500 mg/kg mmo-sat 100 ug/disc (+S9) 致癌性: orl-mus TDLo:135 g/kg/77W-C orl-rat TDLo:164 g/kg/78W-C IARC = 3 (无法对人类的致癌性进行分类)。 NTP =无资料 生殖毒性:无资料 RTECS 号码: AE4200000 模块12. 生态学信息 生态毒性: 鱼类: 96h LC50:>100 mg/L (Oryzias latipes) 甲壳类: 48h EC50:3.5 mg/L (Daphnia magna) 藻类: 72h EC50:150 mg/L (Selenastrum capricornutum) 残留性 / 降解性:无资料 潜在生物累积 (BCF):无资料 土壤中移动性 log水分配系数: 0.49 土壤吸收系数 (Koc):无资料 亨利定律无资料 constant(PaM3/mol): 模块13. 废弃处置 如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中 焚烧。废弃处置时请遵守国家、地区和当地的所有法规。 模块14. 运输信息 联合国分类: 第9类 杂类 UN编号: 3077 正式运输名称: 环境有害物质, 固体, 不另作详细说明 包装等级: III 海洋污染物: Y 模块15. 法规信息 《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、 生产、储存、运输、装卸等方面均作了相应的规定。 4'-羟基乙酰苯胺 修改号码:6 模块16 - 其他信息 N/A |
| 符号 |

GHS07 |
|---|---|
| 信号词 | Warning |
| 危害声明 | H302-H315-H317-H319 |
| 警示性声明 | P280-P301 + P312 + P330-P305 + P351 + P338 |
| 个人防护装备 | dust mask type N95 (US);Eyeshields;Gloves |
| 危害码 (欧洲) | Xn |
| 风险声明 (欧洲) | R22:Harmful if swallowed. R36/37/38:Irritating to eyes, respiratory system and skin . R52/53:Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment . R36/38:Irritating to eyes and skin . R40:Limited evidence of a carci |
| 安全声明 (欧洲) | S26-S36-S61-S37/39 |
| 危险品运输编码 | NONH for all modes of transport |
| WGK德国 | 1 |
| RTECS号 | AE4200000 |
| 海关编码 | 29242930 |
| 对乙酰氨基苯酚上游产品 0 | |
|---|---|
| 对乙酰氨基苯酚下游产品 10 | |
对氨基酚乙酰化而得。方法1:将对氨基酚加入稀乙酸中,再加入冰醋酸,升温至150℃反应7h,加入乙酐,再反应2h,检查终点,合格后冷却至25℃以下,甩滤,水洗至无乙酸味,甩干,得粗品。方法2:将对氨基酚、冰醋酸及含酸50%以上的酸母液一起蒸馏,蒸出稀酸的速度为每小时馏出总量的十分之一,待内温升至130℃以上,取样检查对氨基酚残留量低于2.5%,加入稀酸(含量50%以上),冷却结晶。甩滤,先用少量稀酸洗涤,再用大量水洗至滤液接近无色,得粗品。方法1的收率为90%,方法2的收率为90-95%。精制方法:将水加热至近沸时投入粗品。升温至全溶,加入用水浸泡过的活性炭,用稀乙酸调节至pH=4.2-4.6,沸腾10min。压滤,滤液加少量重亚硫酸钠。冷却至20℃以下,析出结晶。甩滤,水洗,干燥得原料药扑热息痛成品。其他的生产方法还有:(1)在冰醋酸中用锌还原对硝基苯酚,同时乙酰化得到对乙酰氨基酚;(2)将对羟基苯乙酮生成的腙,置于硫酸酸性溶液中,加入亚硝酸钠,转位生成对乙酰氨基酚。
| 海关编码 | 29242930 |
|---|
|
Characterization of a highly sensitive and selective novel trapping reagent, stable isotope labeled glutathione ethyl ester, for the detection of reactive metabolites.
J. Pharmacol. Toxicol. Methods 76 , 83-95, (2015) Glutathione (GSH) trapping assays are widely used to predict the post-marketing risk for idiosyncratic drug reactions (IDRs) in the pharmaceutical industry. Although several GSH derivatives have been ... |
|
|
Application of IL-36 receptor antagonist weakens CCL20 expression and impairs recovery in the late phase of murine acetaminophen-induced liver injury.
Sci. Rep. 5 , 8521, (2015) Overdosing of the analgesic acetaminophen (APAP, paracetamol) is a major cause of acute liver injury. Whereas toxicity is initiated by hepatocyte necrosis, course of disease is regulated by mechanisms... |
|
|
Simple and reliable HPLC method for the monitoring of methotrexate in osteosarcoma patients.
J. Chromatogr. Sci. 52(7) , 590-5, (2014) Methotrexate (MTX) is a dihydrofolate reductase inhibitor that is used for the treatment of tumors and autoimmune diseases. Several automated binding assays are used in clinical practice and numerous ... |
| APAP |
| Calpol |
| NAPAP |
| p-acetaminophenol |
| Panadol |
| Tylenol |
| Exdol |
| 4-(Acetylamino)phenol |
| Fevor |
| N-(p-hydroxyphenyl)acetamide |
| Paracetamol |
| N-(4-Hydroxy-phenyl)-acetamide |
| Korum |
| 4-13-00-01091 (Beilstein Handbook Reference) |
| p-hydroxyacetanilide |
| G 1 |
| p-Acetylaminophenol |
| MFCD00002328 |
| Panex |
| QR DMV1 |
| N-(4-Hydroxyphenyl)acetamide |
| N-Acetyl-4-aminophenol |
| EINECS 203-157-5 |
| 4'-Hydroxyacetanilide |
| N-(4-Hydroxyphenyl)acetanilide |
| Acetaminophen |
| p-(Acetylamino)phenol |
| 4-Acetaminophenol |
| Dirox |
| 4-Acetamidophenol |
| Dypap |
| Hedex |
| N-Acetyl-p-aminophenol |

![N-[4-(2-喹啉甲氧基)苯基]乙酰胺结构式](https://image.chemsrc.com/caspic/180/105326-63-4.png) CAS号105326-63-4
CAS号105326-63-4![[4-[2-(4-azaniumylphenoxy)ethoxy]phenyl]azanium dichloride结构式](https://image.chemsrc.com/caspic/273/109690-44-0.png) CAS号109690-44-0
CAS号109690-44-0 CAS号30824-21-6
CAS号30824-21-6 CAS号3273-78-7
CAS号3273-78-7![N-[4-(羟基乙氧基)苯基]乙酰胺结构式](https://image.chemsrc.com/caspic/174/50375-15-0.png) CAS号50375-15-0
CAS号50375-15-0 CAS号32113-41-0
CAS号32113-41-0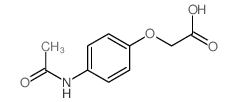 CAS号39149-13-8
CAS号39149-13-8 CAS号52980-20-8
CAS号52980-20-8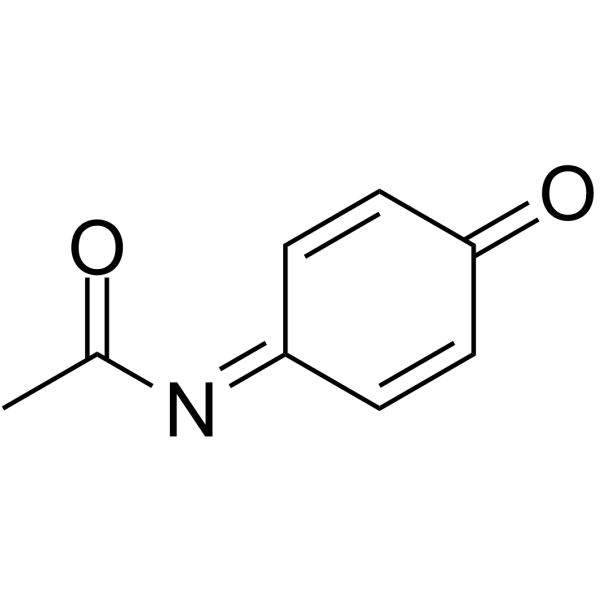 CAS号50700-49-7
CAS号50700-49-7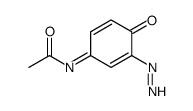 CAS号98555-06-7
CAS号98555-06-7





