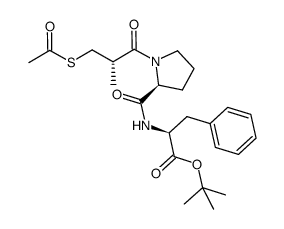
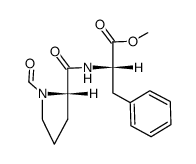
74258-86-9
| 中文名 | 阿拉普利 |
|---|---|
| 英文名 | Alacepril |
| 中文别名 |
2-[[1-(3-乙酰硫基-2-甲基丙酰基)吡咯烷-2-甲酰]氨基]-3-苯丙酸
阿拉普拉 (S)-N-[1-[3-(乙酰基硫代)-2-甲基-1-氧丙基]-L-脯氨酰]-L-苯丙氨酸 |
| 英文别名 |
1-(d-3-acetylthio-2-methylpropanoyl)-l-prolyl-l-phenylalanine
(S)-N-[1-[3-(acetylthio)-2-methyl-1-oxopropyl]-L-prolyl]-L-phenylalanil L-Phenylalanine,1-[(2S)-3-(acetylthio)-2-methyl-1-oxopropyl]-L-prolyl 1-((S)-3-acetylthio-2-methylpropanoyl)-L-prolyl-L-phenylalanine cetapril 1-[(2S)-3-(acetylthio)-2-methyl-1-oxopropyl]-L-prolyl-L-phenylalanine N-[(S)-3-Acetylthio-2-methylpropanoyl]-L-Pro-L-Phe-OH du1219 Cetapfil (s)-l-phenylalanin 1-[(S)-3-Acetylthio-2-methylpropanoyl]-L-Pro-L-Phe-OH MFCD00869538 |
| 描述 | 阿拉西普利(Cetapril)是一种口服活性血管紧张素转换酶(ACE)抑制剂,具有持久的降压作用[1]。 |
|---|---|
| 相关类别 | |
| 体内研究 | 每日连续口服一次(1-2 mg/kg/d)可证实阿拉西普利对肾性高血压大鼠的长期降压作用。在肾性高血压犬中,阿拉西普利(3 mg/kg)表现出稳定和持续的降压作用,其作用时间长于卡托普利[1]。 |
| 参考文献 |
| 密度 | 1.281g/cm3 |
|---|---|
| 沸点 | 679.1ºC at 760 mmHg |
| 分子式 | C20H26N2O5S |
| 分子量 | 406.49600 |
| 闪点 | 364.5ºC |
| 精确质量 | 406.15600 |
| PSA | 129.08000 |
| LogP | 2.03410 |
| 折射率 | 1.581 |
| 储存条件 | -20°C,干燥,密封 |
| 分子结构 | 1、 摩尔折射率:105.81 2、 摩尔体积(cm3/mol):317.3 3、 等张比容(90.2K):873.6 4、 表面张力(dyne/cm):57.4 5、 极化率(10-24cm3):41.94 |
| 更多 | 1.性状:从乙醇-n-己烷结晶,白色结晶或结晶性粉末,无气味或略有异味,味微苦。 2.熔点(℃):155-156。 3.比旋光度(C=1.02,乙醇)):-81.3°。 4.溶解度:易溶于氯仿或甲醇,较易溶于乙醇,较难溶于丙酮,难溶于水,极难溶于乙醚 |
|
毒理学数据: 急性毒性LD50大鼠,小鼠(mg/kg):>5000,>5000口服;>3000,>3000皮下注射;约2000,约3000腹腔注射。 CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| 危害码 (欧洲) | Xn |
|---|---|
| 风险声明 (欧洲) | R22:Harmful if swallowed. |
| WGK德国 | 3 |
| RTECS号 | UO3516500 |
|
~99% 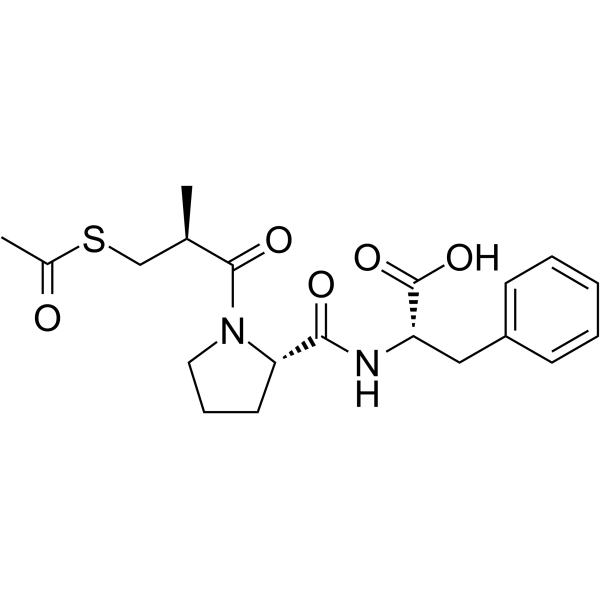
74258-86-9 |
| 文献:NICOX S.A. Patent: WO2004/106300 A1, 2004 ; Location in patent: Page 65 ; |
|
~% 
74258-86-9 |
| 文献:US4248883 A1, ; |
|
~65% 
74258-86-9 |
| 文献:Sawayama; Itokawa; Shimada; Doi; Kimura; Nishim ura Chemical and Pharmaceutical Bulletin, 1990 , vol. 38, # 2 p. 529 - 531 |
|
~% 
74258-86-9 |
| 文献:Chemical and Pharmaceutical Bulletin, , vol. 38, # 2 p. 529 - 531 |
|
~% 
74258-86-9 |
| 文献:Chemical and Pharmaceutical Bulletin, , vol. 38, # 2 p. 529 - 531 |
|
~% 
74258-86-9 |
| 文献:Chemical and Pharmaceutical Bulletin, , vol. 38, # 2 p. 529 - 531 |
|
~% 
74258-86-9 |
| 文献:Chemical and Pharmaceutical Bulletin, , vol. 38, # 2 p. 529 - 531 |
| 上游产品 7 | |
|---|---|
| 下游产品 0 | |