γ-丁内酯
一般危化品
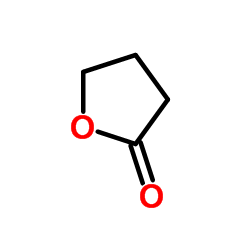
γ-丁内酯结构式
|
常用名 | γ-丁内酯 | 英文名 | gamma-Butyrolactone |
|---|---|---|---|---|
| CAS号 | 96-48-0 | 分子量 | 86.089 | |
| 密度 | 1.1±0.1 g/cm3 | 沸点 | 204.0±0.0 °C at 760 mmHg | |
| 分子式 | C4H6O2 | 熔点 | −45 °C(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 98.3±0.0 °C | |
| 符号 |


GHS05, GHS07 |
信号词 | Danger |
|
An improved method for the analysis of GHB in human hair by liquid chromatography tandem mass spectrometry.
J. Anal. Toxicol. 39(2) , 83-8, (2015) The abuse of gamma-hydroxybutyric acid (GHB) and its suspicion in cases of suspected drug-facilitated sexual assault is of keen interest to forensic toxicology laboratories. This paper reports an extraction, separation and detection procedure for GHB in hair ... |
|
|
Volatile Compounds from Grape Skin, Juice and Wine from Five Interspecific Hybrid Grape Cultivars Grown in Québec (Canada) for Wine Production.
Molecules 20 , 10980-1016, (2015) Developed from crosses between Vitis vinifera and North American Vitis species, interspecific hybrid grape varieties are becoming economically significant in northern areas, where they are now extensively grown for wine production. However, the varietal diffe... |
|
|
Polyhydroxyalkanoates: waste glycerol upgrade into electrospun fibrous scaffolds for stem cells culture.
Int. J. Biol. Macromol. 71 , 131-40, (2014) This integrated study shows that waste glycerol can be bio-valorized by the fabrication of electrospun scaffolds for stem cells. Human mesenchymal stem cells (hMSC) provide an interesting model of regenerating cells because of their ability to differentiate i... |
|
|
A platform pathway for production of 3-hydroxyacids provides a biosynthetic route to 3-hydroxy-γ-butyrolactone.
Nat. Commun. 4 , 1414, (2013) The replacement of petroleum feedstocks with biomass to produce platform chemicals requires the development of appropriate conversion technologies. 3-Hydroxy-γ-butyrolactone has been identified as one such chemical; however, there are no naturally occurring b... |
|
|
Barrier Function of the Repaired Skin Is Disrupted Following Arrest of Dicer in Keratinocytes.
Mol. Ther. 23 , 1201-10, (2015) Tissue injury transiently silences miRNA-dependent posttranscriptional gene silencing in its effort to unleash adult tissue repair. Once the wound is closed, miRNA biogenesis is induced averting neoplasia. In this work, we report that Dicer plays an important... |
|
|
Comparative safety of the antifouling compound butenolide and 4,5-dichloro-2-n-octyl-4-isothiazolin-3-one (DCOIT) to the marine medaka (Oryzias melastigma).
Aquat. Toxicol. 149 , 116-25, (2014) This study evaluated the potential adverse effects of butenolide, a promising antifouling compound, using the marine medaka (Oryzias melastigma), a model fish for marine ecotoxicology. The active ingredient used in the commercial antifoulant SeaNine 211, 4,5-... |
|
|
Proteomic changes in brain tissues of marine medaka (Oryzias melastigma) after chronic exposure to two antifouling compounds: butenolide and 4,5-dichloro-2-n-octyl-4-isothiazolin-3-one (DCOIT).
Aquat. Toxicol. 157 , 47-56, (2014) SeaNine 211 with active ingredient of 4,5-dichloro-2-n-octyl-4-isothiazolin-3-one (DCOIT) has been used as a "green" antifouling agent worldwide but has raised serious biosafety concerns in coastal environments. DCOIT has the potential to disrupt the neurotra... |
|
|
Alpha, beta-unsaturated lactones 2-furanone and 2-pyrone induce cellular DNA damage, formation of topoisomerase I- and II-DNA complexes and cancer cell death.
Toxicol. Lett. 222(1) , 64-71, (2013) The alpha, beta-unsaturated lactones 2-furanone and 2-pyrone are part of the chemical structure of a variety of naturally occurring compounds (e.g., cardenolides, bufadienolides, acetogenins, coumarins, and food-flavoring furanones), some of which have shown ... |
|
|
Characterization of the PON1 active site using modeling simulation, in relation to PON1 lactonase activity
Bioorg. Med. Chem. 16 , 7504-9, (2008) Paraoxonase1 (PON1) is a HDL bound enzyme and many of the anti-atherogenic properties of HDL are attributed to PON1. The enzyme precise mechanism of protective action and its endogenous substrate remain elusive. PON1 hydrolyzes organophosphates, arylesters an... |
|
|
Catalytic asymmetric synthesis of γ-butenolides by direct vinylogous reactions.
Mini Rev. Med. Chem. 13(6) , 845-53, (2013) The γ-butenolide structural motif is a prominent feature in many bioactive natural products and drugs. This short review summarizes catalytic asymmetric synthesis of γ-butenolides through direct vinylogous reactions by metal complexes and organocatalysts. In ... |