5-乙基-5-苯基咪唑烷-2,4-二酮
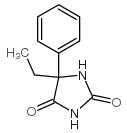
5-乙基-5-苯基咪唑烷-2,4-二酮结构式

|
常用名 | 5-乙基-5-苯基咪唑烷-2,4-二酮 | 英文名 | 2,4-Imidazolidinedione,5-ethyl-5-phenyl |
|---|---|---|---|---|
| CAS号 | 631-07-2 | 分子量 | 204.22500 | |
| 密度 | 1.173 g/cm3 | 沸点 | N/A | |
| 分子式 | C11H12N2O2 | 熔点 | 195-200ºC | |
| MSDS | 美版 | 闪点 | N/A | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Pharmacodynamics of cytochrome P450 2B induction by phenobarbital, 5-ethyl-5-phenylhydantoin, and 5-ethyl-5-phenyloxazolidinedione in the male rat liver or in cultured rat hepatocytes.
Chem. Res. Toxicol. 6(2) , 188-96, (1993) The pharmacodynamics of rat hepatic cytochrome P450 2B (P450 2B) induction by phenobarbital (PB) and two structural congeners, dl-5-ethyl-5-phenylhydantoin (EPH) and dl-5-ethyl-5-phenyloxazolidinedione (EPO), were investigated. The in vivo induction of P450 2... |
|
|
A markedly diminished pleiotropic response to phenobarbital and structurally-related xenobiotics in Zucker rats in comparison with F344/NCr or DA rats.
Biochem. Pharmacol. 43(5) , 1079-87, (1992) Phenobarbital (PB) and certain structurally-related compounds induce a variety of hepatic drug-metabolizing enzymes in many strains of rats. Thus, following administration of PB (300, 500 ppm), barbital (BB, 1500 ppm) or 5-ethyl-5-phenylhydantoin (EPH, 500 pp... |
|
|
Active-site characteristics of CYP2C19 and CYP2C9 probed with hydantoin and barbiturate inhibitors.
Arch. Biochem. Biophys. 429(1) , 1-15, (2004) Three series of N-3 alkyl substituted phenytoin, nirvanol, and barbiturate derivatives were synthesized and their inhibitor potencies were tested against recombinant CYP2C19 and CYP2C9 to probe the interaction of these ligands with the active sites of these e... |
|
|
Electrochemical determination of purine and pyrimidine DNA bases based on the recognition properties of azocalix[4]arene.
J. Biochem. Toxicol. 9(5) , 269-78, (1994) The azocalix[4]arene film modified glassy carbon electrode was established for the convenient and sensitive detection of four DNA bases (guanine, adenine, thymine and cytosine). Field emission scanning electron microscopy, attenuated total reflectance-FTIR an... |
|
|
Biopharmaceutical studies on hydantoin derivatives. V. Pharmacokinetics and pharmacodynamics of 5,5-diphenylhydantoin and 1-benzenesulfonyl-5,5-diphenylhydantoin.
J. Pharmacobiodyn. 9(3) , 303-14, (1986) Disposition of 1-benzenesulfonyl-5,5-diphenylhydantoin (II) having a potent anti-inflammatory activity was compared with that of 5,5-diphenylhydantoin (I), an antiepileptic drug, in order to elucidate whether the pharmacodynamic difference between them can be... |
|
|
A comparison of the S(+) and R(-) enantiomers of 5-ethyl-5-phenylhydantoin as hypolipidemic agents in rodents.
Biomed. Biochim. Acta 46(7) , 623-34, (1987) An investigation of the effects of the S-(+) and R-(-) enantiomers of 5-ethyl-5-phenylhydantoin as hypolipidemic agents was undertaken. The serum cholesterol and triglyceride levels were reduced approximately 40% by either oral or i.p. administration at 20 mg... |
|
|
Quantification of mephenytoin and its metabolites 4'-hydroxymephenytoin and nirvanol in human urine using a simple sample processing method.
Rapid Commun. Mass Spectrom. 18(15) , 1675-80, (2004) A reliable and easy to use liquid chromatography/tandem mass spectrometry (LC/MS/MS) method without the use of sample extraction was developed for the simultaneous quantification of urinary concentrations of mephenytoin, a standard phenotyping substrate for t... |
|
|
Drug-metal interactions: spectroscopic studies of copper hydantoin complexes.
J. Inorg. Biochem. 42(4) , 267-72, (1991) The preparation and spectral properties of copper(II) complexes of two hydantoins are reported. Complexes of the general formula Cu(hyd)2(py)2, where hyd = phenytoin or nirvanol; and py = pyridine were prepared and characterized by infrared and ESR. Spectral ... |
|
|
Assessment of urinary mephenytoin metrics to phenotype for CYP2C19 and CYP2B6 activity.
Eur. J. Clin. Pharmacol. 64(4) , 387-98, (2008) (S)-Mephenytoin is selectively metabolised to (S)-4'-hydroxymephenytoin by CYP2C19. The urinary excretion of 4'-hydroxymephenytoin reflects the activity of individual enzymes. We evaluated fractioned urinary collection and beta-glucuronidase pre-treatment in ... |
|
|
Mephenytoin stereoselective elimination in the rat: II. Comparison of mephenytoin stereoselective clearance during chronic intravenous and hepatic portal vein administration.
Eur. J. Drug Metab. Pharmacokinet. 14(4) , 269-78, (1989) The stereoselective clearances of R- and S-mephenytoin were determined in rats receiving either an intravenous or hepatic portal vein infusion of racemic mephenytoin. The mean +/- SD intravenous clearances of R- and S-mephenytoin were 1630 +/- 250 ml/hr and 6... |