琥珀酰胺
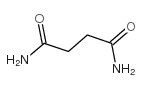
琥珀酰胺结构式

|
常用名 | 琥珀酰胺 | 英文名 | Butanediamide |
|---|---|---|---|---|
| CAS号 | 110-14-5 | 分子量 | 116.11900 | |
| 密度 | 1.207 g/cm3 | 沸点 | 494ºC at 760 mmHg | |
| 分子式 | C4H8N2O2 | 熔点 | 260-265 °C (dec.)(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 252.6ºC | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Effects of structural analogues of the substrate and allosteric regulator of the human mitochondrial NAD(P)+-dependent malic enzyme.
Bioorg. Med. Chem. 17 , 5414-9, (2009) Fumarate, a four-carbon trans dicarboxylic acid, is the allosteric activator of the human mitochondrial NAD(P)(+)-dependent malic enzyme (m-NAD(P)-ME). In this paper, we discuss the effects of the structural analogues of fumarate on human m-NAD(P)-ME. Succina... |
|
|
Synthesis of novel melanocortin 4 receptor agonists and antagonists containing a succinamide core.
Bioorg. Med. Chem. Lett. 14 , 377, (2004) A novel series of piperazines appended to a succinamide backbone were synthesized and found to have a high affinity for the melanocortin-4 receptor (IC(50)s ranging from <0.1 to 200 nM). Both agonists and antagonists of MC4R were prepared by modifying the gro... |
|
|
A disubstituted succinamide is a potent sodium channel blocker with efficacy in a rat pain model.
Biochemistry 43(30) , 9866-76, (2004) Sodium channel blockers are used clinically to treat a number of neuropathic pain conditions, but more potent and selective agents should improve on the therapeutic index of currently used drugs. In a high-throughput functional assay, a novel sodium channel (... |
|
|
Structure analysis reveals the flexibility of the ADAMTS-5 active site.
Protein Sci. 20(4) , 735-44, (2011) A ((1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl) succinamide derivative (here referred to as Compound 12) shows significant activity toward many matrix metalloproteinases (MMPs), including MMP-2, MMP-8, MMP-9, and MMP-13. Modeling studies had predicted that th... |
|
|
Adaptable synthesis of C-glycosidic multivalent carbohydrates and succinamide-linked derivatization.
Org. Lett. 12(22) , 5262-5, (2010) A modular approach to the synthesis of trivalent C-glycosidic carbohydrates is described. The approach is illustrated employing carboxylate-terminated C-glycosidic d-mannose, d-glucose, and d-galactose derivatives with different length C1-linked spacer units ... |
|
|
Synthesis of novel succinamide derivatives having the 5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one skeleton as potent and selective M2 muscarinic receptor antagonists. I.
Chem. Pharm. Bull. 45(6) , 996-1007, (1997) A series of 5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one derivatives containing the succinamide skeleton has been synthesized and evaluated for M1, M2 and M3 muscarinic receptor binding affinities (in vitro) and M2 and M3 muscarinic receptor antagoni... |
|
|
Synthesis of novel succinamide derivatives having a 5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one skeleton as potent and selective M2 muscarinic receptor antagonists. II.
Chem. Pharm. Bull. 45(9) , 1458-69, (1997) A series of succinamide derivatives containing the 5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one skeleton (6a-z) was prepared and evaluated for binding affinity to muscarinic receptors in vitro and for antagonism of bradycardia and salivation in vivo ... |
|
|
Discovery of selective metal-binding peptoids using 19F encoded combinatorial libraries.
Bioorg. Med. Chem. Lett. 10(18) , 2115-8, (2000) A method for encoding solid-phase split/mix combinatorial libraries using the chemical shift of synthetic fluoroarenes ('F-codes') has been developed. They have wide chemical shift dispersion and are detectable at the sub-micromol level. 19F NMR is used for d... |
|
|
Novel P2-P4 macrocyclic inhibitors of HCV NS3/4A protease by P3 succinamide fragment depeptidization strategy.
Bioorg. Med. Chem. Lett. 20(1) , 168-74, (2010) Hepatitis C represents a serious worldwide health-care problem. Recently, we have disclosed a novel class of P2-P4 macrocyclic inhibitors of NS3/4A protease containing a carbamate functionality as capping group at the P3 N-terminus. Herein we report our work ... |
|
|
Synthesis and SAR of succinamide peptidomimetic inhibitors of cathepsin S.
Bioorg. Med. Chem. Lett. 17(10) , 2899-903, (2007) Peptidic, non-covalent inhibitors of lysosomal cysteine protease cathepsin S (1 and 2) were investigated due to low oral bioavailability, leading to an improved series of peptidomimetic inhibitors. Utilizing phenyl succinamides as the P2 residue increased the... |