Structure analysis reveals the flexibility of the ADAMTS-5 active site.
Huey-Sheng Shieh, Alfredo G Tomasselli, Karl J Mathis, Mark E Schnute, Scott S Woodard, Nicole Caspers, Jennifer M Williams, James R Kiefer, Grace Munie, Arthur Wittwer, Anne-Marie Malfait, Micky D Tortorella
文献索引:Protein Sci. 20(4) , 735-44, (2011)
全文:HTML全文
摘要
A ((1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl) succinamide derivative (here referred to as Compound 12) shows significant activity toward many matrix metalloproteinases (MMPs), including MMP-2, MMP-8, MMP-9, and MMP-13. Modeling studies had predicted that this compound would not bind to ADAMTS-5 (a disintegrin and metalloproteinase with thrombospondin motifs-5) due to its shallow S1' pocket. However, inhibition analysis revealed it to be a nanomolar inhibitor of both ADAMTS-4 and -5. The observed inconsistency was explained by analysis of crystallographic structures, which showed that Compound 12 in complex with the catalytic domain of ADAMTS-5 (cataTS5) exhibits an unusual conformation in the S1' pocket of the protein. This first demonstration that cataTS5 can undergo an induced conformational change in its active site pocket by a molecule like Compound 12 should enable the design of new aggrecanase inhibitors with better potency and selectivity profiles.Copyright © 2011 The Protein Society.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
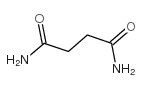 |
琥珀酰胺
CAS:110-14-5 |
C4H8N2O2 |
|
Effects of structural analogues of the substrate and alloste...
2009-08-01 [Bioorg. Med. Chem. 17 , 5414-9, (2009)] |
|
Synthesis of novel melanocortin 4 receptor agonists and anta...
2004-01-19 [Bioorg. Med. Chem. Lett. 14 , 377, (2004)] |
|
A disubstituted succinamide is a potent sodium channel block...
2004-08-03 [Biochemistry 43(30) , 9866-76, (2004)] |
|
Adaptable synthesis of C-glycosidic multivalent carbohydrate...
2010-11-19 [Org. Lett. 12(22) , 5262-5, (2010)] |
|
Synthesis of novel succinamide derivatives having the 5,11-d...
1997-06-01 [Chem. Pharm. Bull. 45(6) , 996-1007, (1997)] |