DL-3,4-二羟基杏仁酸
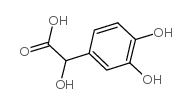
DL-3,4-二羟基杏仁酸结构式

|
常用名 | DL-3,4-二羟基杏仁酸 | 英文名 | 3,4-Dihydroxymandelic acid |
|---|---|---|---|---|
| CAS号 | 14883-87-5 | 分子量 | 184.14600 | |
| 密度 | 1.644g/cm3 | 沸点 | 487.5ºC at 760 mmHg | |
| 分子式 | C8H8O5 | 熔点 | 136-137 °C (dec.)(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 262.7ºC | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Lipophilicity of amine neurotransmitter precursors, metabolites and related drugs estimated on various TLC plates.
J. Chromatogr. Sci. 52(9) , 1095-103, (2014) The retention behavior for a series of amine neurotransmitters, their precursors, metabolites and structurally related drugs has been investigated in reversed-phase thin-layer chromatography using RP-18, RP-8, RP-2, CN and Diol stationary phases and mixtures ... |
|
|
Further study on the use of uncharged beta-cyclodextrin polymer in capillary electrophoresis: enantiomeric separation of some alpha-hydroxy acids.
Electrophoresis 16(8) , 1505-9, (1995) Uncharged beta-cyclodextrin polymer was used as chiral selector for the enantiomeric separation of some alpha-hydroxy acids by capillary electrophoresis. Complexation and enantiomeric resolution of mandelic acid, m-hydroxy and p-hydroxymandelic acid, 3,4-dihy... |
|
|
Mechanistic studies on tyrosinase-catalysed oxidative decarboxylation of 3,4-dihydroxymandelic acid.
Biochem. J. 281 ( Pt 2) , 353-7, (1992) Mushroom tyrosinase, which is known to convert a variety of o-diphenols into o-benzoquinones, has been shown to catalyse an unusual oxidative decarboxylation of 3,4-dihydroxymandelic acid to 3,4-dihydroxybenzaldehyde [Sugumaran (1986) Biochemistry 25, 4489-44... |
|
|
Chemical and enzymic oxidation by tyrosinase of 3,4-dihydroxymandelate.
Biochem. J. 256(2) , 681-4, (1988) Tyrosinase usually catalyses the conversion of monophenols into o-diphenols and the oxidation of diphenols to the corresponding o-quinones. Sugumaran [(1986) Biochemistry 25, 4489-4492] has previously proposed an unusual oxidative decarboxylation of 3,4-dihyd... |
|
|
Oxidative decarboxylation of 3,4-dihydroxymandelic acid to 3,4-dihydroxybenzaldehyde: electrochemical and HPLC analysis of the reaction mechanism.
Biochim. Biophys. Acta 1077(3) , 400-6, (1991) Cyclic voltammetric and chronoamperometric data are consistent with a process in which 3,4-dihydroxymandelic acid (DOMA) is oxidized initially in a two-electron step to its corresponding o-benzoquinone. This species is unstable and undergoes the rate-determin... |
|
|
3,4-Dihydroxymandelic acid, a noradrenalin metabolite with powerful antioxidative potential.
J. Agric. Food Chem. 50(21) , 5897-902, (2002) The decarboxylated noradrenaline metabolite 3,4-dihydroxymandelic acid [DHMA, 2-(3,4-dihydroxyphenyl)-2-hydroxyacetic acid] occurs in different mammalian tissues, especially in the heart. To elucidate the physiological function of DHMA, the antioxidative and ... |
|
|
The role of MAO-A and MAO-B in the metabolic degradation of noradrenaline in human arteries.
J. Auton. Pharmacol. 18 , 123-128, (1998) 1. Segments of human cystic, gastric and ileocolic arteries were obtained from patients undergoing surgery. 2. Segments of arterial tissues, the noradrenaline content of which ranged between 0.27 and 0.52 microg g(-1), were incubated with 0.1 micromol l(-1) [... |
|
|
Electrochemical determination of diphenol oxidase activity using high-pressure liquid chromatography.
Anal. Biochem. 190(2) , 354-9, (1990) A quantitative assay for the diphenol oxidase activity of tyrosinase (EC 1.14.18.1) using high-pressure liquid chromatography with electrochemical detection is described. The assay is based on the observation (M. Sugumaran, 1986, Biochemistry 25, 4489-4492) t... |
|
|
The influence of dietary restriction, germ-free status, and aging on adrenal catecholamines in Lobund-Wistar rats.
J. Gerontol. 46(4) , B135-41, (1991) Adrenal catecholamines (CA) were measured in 6-, 18-, and 30-mo Lobund-Wistar rats (LWR) maintained under germ-free or conventional conditions and fed either ad libitum or a restricted (70% of adult ad libitum) diet. Levels of dopamine (DA), norepinephrine (N... |
|
|
Analysis of acidic metabolites of biogenic amines in bovine retina and vitreous and aqueous humour by gas chromatography-negative ion chemical ionisation mass spectrometry.
J. Neurochem. 55(3) , 842-8, (1990) Acidic metabolites of a number of biogenic amines have been identified and quantified by reaction with either acetic or propionic anhydride in the aqueous phase followed by extraction into ethyl acetate, esterification of carboxyl groups with ditrifluoromethy... |