Chemical and enzymic oxidation by tyrosinase of 3,4-dihydroxymandelate.
J Cabanes, A Sanchez-Ferrer, R Bru, F García-Carmona
文献索引:Biochem. J. 256(2) , 681-4, (1988)
全文:HTML全文
摘要
Tyrosinase usually catalyses the conversion of monophenols into o-diphenols and the oxidation of diphenols to the corresponding o-quinones. Sugumaran [(1986) Biochemistry 25, 4489-4492] has previously proposed an unusual oxidative decarboxylation of 3,4-dihydroxymandelate catalysed by tyrosinase. Our determination of the intermediates involved in the reaction demonstrated that 3,4-dihydroxybenzaldehyde is not the first intermediate appearing in the medium during the enzymic reaction. Re-examination of this new activity of tyrosinase has demonstrated that the product of the enzyme action is the o-quinone, which, owing to its instability, evolves to the final product, 3,4-dihydroxybenzaldehyde, by a chemical reaction of oxidative decarboxylation.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
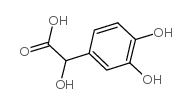 |
DL-3,4-二羟基杏仁酸
CAS:14883-87-5 |
C8H8O5 |
|
Lipophilicity of amine neurotransmitter precursors, metaboli...
2014-10-01 [J. Chromatogr. Sci. 52(9) , 1095-103, (2014)] |
|
Further study on the use of uncharged beta-cyclodextrin poly...
1995-08-01 [Electrophoresis 16(8) , 1505-9, (1995)] |
|
Mechanistic studies on tyrosinase-catalysed oxidative decarb...
1992-01-15 [Biochem. J. 281 ( Pt 2) , 353-7, (1992)] |
|
Oxidative decarboxylation of 3,4-dihydroxymandelic acid to 3...
1991-04-29 [Biochim. Biophys. Acta 1077(3) , 400-6, (1991)] |
|
3,4-Dihydroxymandelic acid, a noradrenalin metabolite with p...
2002-10-09 [J. Agric. Food Chem. 50(21) , 5897-902, (2002)] |