硫代苯甲酰胺
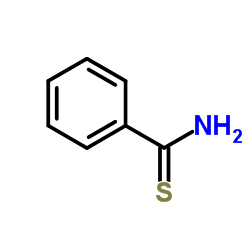
硫代苯甲酰胺结构式

|
常用名 | 硫代苯甲酰胺 | 英文名 | Thiobenzamide |
|---|---|---|---|---|
| CAS号 | 2227-79-4 | 分子量 | 137.202 | |
| 密度 | 1.2±0.1 g/cm3 | 沸点 | 245.0±23.0 °C at 760 mmHg | |
| 分子式 | C7H7NS | 熔点 | 113-117 °C(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 102.0±22.6 °C | |
| 符号 |

GHS06 |
信号词 | Danger |
|
Cytotoxic activity of 3-(5-phenyl-3H-[1,2,4]dithiazol-3-yl)chromen-4-ones and 4-oxo-4H-chromene-3-carbothioic acid N-phenylamides.
Eur. J. Med. Chem. 45 , 790-4, (2010) 6/6,7-Substituted-3-formylchromones (8a-g) were reacted with 2 equivalents thiobenzamide (9) in refluxing toluene to furnish substituted-3-(5-phenyl-3H-[1,2,4]dithiazol-3-yl)chromen-4-ones (10a-g) in high yields. Similarly, when substituted-2-anilino-3-formyl... |
|
|
Potent inhibition of alcohol self-administration in alcohol-preferring rats by a κ-opioid receptor antagonist.
J. Pharmacol. Exp. Ther. 350(1) , 171-80, (2014) A substituted aryl amide derivative of 6-naltrexamine--17-cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-[(4'-trimethylfluoro)benzamido]morphinan-hydrochloride--(compound 5), previously shown to be a potent κ-opioid receptor antagonist, was used to character... |
|
|
Facile synthesis of nitriles via manganese oxide promoted oxidative dehydrosulfurization of primary thioamides.
Chem. Commun. (Camb.) 48(91) , 11247-9, (2012) In the presence of manganese oxides, dehydrosulfurization of various kinds of primary thioamides including aromatic, heterocyclic, and aliphatic ones efficiently proceeded to give the corresponding nitriles in high yields. The observed catalysis was truly het... |
|
|
Hydrogen sulfide enhances ulcer healing in rats.
FASEB J. 21 , 4070-4076, (2007) Hydrogen sulfide is an endogenous mediator that relaxes vascular smooth muscle, exhibits several antiinflammatory activities, and contributes to gastric mucosal defense. This study was performed to examine the role of hydrogen sulfide in the resolution of inj... |
|
|
Effects of the herbicide chlorthiamid on the olfactory mucosa.
Toxicol. Lett. 76(3) , 203-8, (1995) Chlorthiamid (2,6-dichlorothiobenzamide) and its major metabolite 2,6-dichlorobenzonitrile are olfactory toxicants with a high in vivo covalent binding in the olfactory mucosa of mice. This study showed that the cytochrome P450 (P450) inhibitors, metyrapone a... |
|
|
Placental peroxidase--further purification of the enzyme and oxidation of thiobenzamide.
Placenta 14(3) , 309-19, (1993) Human term placental peroxidase [donor: hydrogen peroxide (H2O2) oxidoreductase] from non-smoking women was purified by extraction of the membrane fraction with 0.5 M Ca2+ followed by affinity chromatography on concanavalin A, hydrophobic chromatography on ph... |
|
|
Structure-activity relationships for a series of thiobenzamide influenza fusion inhibitors derived from 1,3,3-trimethyl-5-hydroxy-cyclohexylmethylamine.
Bioorg. Med. Chem. Lett. 12(23) , 3379-82, (2002) The anti-influenza activity of a series of thiobenzamide fusion inhibitors derived from 1,3,3-trimethyl-5-hydroxy-cyclohexylmethylamine is profiled. Axial disposition of the thioamide moiety is essential for potent influenza inhibitory activity. |
|
|
Peroxidase-catalyzed S-oxygenation: mechanism of oxygen transfer for lactoperoxidase.
Biochemistry 30(37) , 8960-4, (1991) The mechanism of organosulfur oxygenation by peroxidases [lactoperoxidase (LPX), chloroperoxidase, thyroid peroxidase, and horseradish peroxidase] and hydrogen peroxide was investigated by use of para-substituted thiobenzamides and thioanisoles. The rate cons... |
|
|
Metabolism of thioamides by Ralstonia pickettii TA.
Appl. Environ. Microbiol. 72(12) , 7468-76, (2006) Information on bacterial thioamide metabolism has focused on transformation of the antituberculosis drug ethionamide and related compounds by Mycobacterium tuberculosis. To study this metabolism more generally, a bacterium that grew using thioacetamide as the... |
|
|
Relative hepatotoxicity of 2-(substituted phenyl)thiazoles and substituted thiobenzamides in mice: evidence for the involvement of thiobenzamides as ring cleavage metabolites in the hepatotoxicity of 2-phenylthiazoles.
Toxicol. Lett. 85(2) , 101-5, (1996) The hepatotoxicity of the 3 isomers of para-substituted thiobenzamides and the 3 isomers of 2-(para-substituted phenyl)-4-methylthiazoles was evaluated in mice depleted of glutathione (GSH) by pretreatment with buthionine sulfoximine (BSO). In accordance with... |