Placental peroxidase--further purification of the enzyme and oxidation of thiobenzamide.
P Joseph, N S Srinivasan, A P Kulkarni
文献索引:Placenta 14(3) , 309-19, (1993)
全文:HTML全文
摘要
Human term placental peroxidase [donor: hydrogen peroxide (H2O2) oxidoreductase] from non-smoking women was purified by extraction of the membrane fraction with 0.5 M Ca2+ followed by affinity chromatography on concanavalin A, hydrophobic chromatography on phenyl sepharose CL-4B and gel filtration chromatography on sephacryl S-200 columns. The final enzyme preparation was 110-fold pure when compared to the Ca2+ extract and the overall recovery of the procedure was 55 per cent. SDS-PAGE of the final product showed the presence of four protein staining bands of molecular weights 30, 32.5, 35 and 55 KDa. The purified peroxidase eluted as a single peak from the sephacryl S-200 column suggesting apparent homogeneity of the protein. The purified placental peroxidase oxidized thiobenzamide to its sulfoxide. The highest rate of TB S-oxidation under optimum conditions was 18 +/- 2.15 mumoles/min/mg protein. The H2O2-dependent thiobenzamide S-oxidation catalysed by the placental peroxidase was inhibited by KCN and NaN3 with IC50 values of 16.9 and 34 microM respectively.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
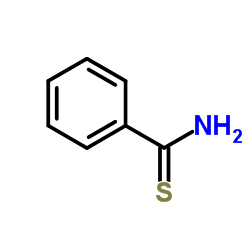 |
硫代苯甲酰胺
CAS:2227-79-4 |
C7H7NS |
|
Cytotoxic activity of 3-(5-phenyl-3H-[1,2,4]dithiazol-3-yl)c...
2010-02-01 [Eur. J. Med. Chem. 45 , 790-4, (2010)] |
|
Potent inhibition of alcohol self-administration in alcohol-...
2014-07-01 [J. Pharmacol. Exp. Ther. 350(1) , 171-80, (2014)] |
|
Facile synthesis of nitriles via manganese oxide promoted ox...
2012-11-25 [Chem. Commun. (Camb.) 48(91) , 11247-9, (2012)] |
|
Hydrogen sulfide enhances ulcer healing in rats.
2007-12-01 [FASEB J. 21 , 4070-4076, (2007)] |
|
Effects of the herbicide chlorthiamid on the olfactory mucos...
1995-04-01 [Toxicol. Lett. 76(3) , 203-8, (1995)] |