2-溴-1,4-萘并醌
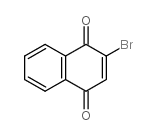
2-溴-1,4-萘并醌结构式

|
常用名 | 2-溴-1,4-萘并醌 | 英文名 | 1,4-Naphthalenedione,2-bromo- |
|---|---|---|---|---|
| CAS号 | 2065-37-4 | 分子量 | 237.04900 | |
| 密度 | 1.757g/cm3 | 沸点 | 324.2ºC at 760mmHg | |
| 分子式 | C10H5BrO2 | 熔点 | 131-133ºC(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 124.7ºC | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Potent naphthoquinones against antimony-sensitive and -resistant Leishmania parasites: synthesis of novel α- and nor-α-lapachone-based 1,2,3-triazoles by copper-catalyzed azide-alkyne cycloaddition.
Eur. J. Med. Chem. 63 , 523-30, (2013) Continuing our screening program for novel anti-parasite compounds, we synthesized seven 1,4-naphthoquinones coupled to 1,2,3-triazoles, five nor-β-lapachone-based 1,2,3-triazoles and ten α-lapachone-based 1,2,3-triazoles. These and other naphthoquinonoid com... |
|
|
2-Bromo-1,4-naphthoquinone: a potentially improved substitute of menadione in Apatone™ therapy.
Braz. J. Med. Biol. Res. 45(8) , 701-10, (2012) Apatone™, a combination of menadione (2-methyl-1,4-naphthoquinone, VK3) and ascorbic acid (vitamin C, VC) is a new strategy for cancer treatment. Part of its effect on tumor cells is related to the cellular pro-oxidative imbalance provoked by the generation o... |
|
|
Expedient, one-pot preparation of fused indoles via CAN-catalyzed three-component domino sequences and their transformation into polyheterocyclic compounds containing pyrrolo[1,2-a]azepine fragments.
Org. Biomol. Chem. 8(15) , 3426-36, (2010) The CAN-catalyzed three-component between reaction between primary amines, beta-dicarbonyl compounds and naphthoquinones or 2-bromonaphthoquinones afforded, respectively, 5-hydroxybenzo[g]indoles and benzo[f]indole-4,9-diones, the former of which were transfo... |
|
|
Reaction of Some Indoles with 1,4-Naphthoquinones in the Presence of Pd(OAc)2. Tanoue Y, et al.
J. Heterocycl. Chem. 51(S1) , E364, (2014)
|
|
|
Stereoselective synthesis of deoxy analogues of the 3C-protease inhibitor thysanone. Brimble MA and Elliott RJR
Tetrahedron 58(1) , 183-189, (2002)
|
|
|
The Photochemical Reaction of 1,4-Naphthoquinone Derivatives with Hydrogen Donors. Maruyama K and Arakawa S.
Bull. Chem. Soc. Jpn. 47(8) , 1960-1966, (1974)
|