丙二酸环(亚)异丙酯
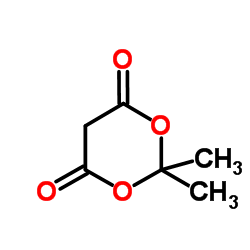
丙二酸环(亚)异丙酯结构式

|
常用名 | 丙二酸环(亚)异丙酯 | 英文名 | Meldrumic acid |
|---|---|---|---|---|
| CAS号 | 2033-24-1 | 分子量 | 144.13 | |
| 密度 | 1.2±0.1 g/cm3 | 沸点 | 356.7±35.0 °C at 760 mmHg | |
| 分子式 | C6H8O4 | 熔点 | 92-96 °C(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 195.1±24.4 °C |
|
Synthesis and biological evaluation of arylidene analogues of Meldrum's acid as a new class of antimalarial and antioxidant agents.
Bioorg. Med. Chem. 18 , 5626-33, (2010) A series of arylidene analogues of Meldrum's acid were synthesized and evaluated for in vitro antimalarial and antioxidant activities for the first time. The influence of various physico-chemical parameters such as dielectric constant (epsilon), donor number ... |
|
|
One hundred years of Meldrum's acid: advances in the synthesis of pyridine and pyrimidine derivatives.
Mol. Divers. 13(4) , 399-419, (2009) A general review (138 references) focused on the recent advances in the application of Meldrum's acid reactivity for synthesis of diverse pyridine and pyrimidine derivatives, mostly small and drug-like molecules is presented. |
|
|
A new domino autocatalytic reaction leading to polyfunctionalized spiro[5.5]undecanes and dispiro[4.2.5.2]pentadecanes.
Org. Biomol. Chem. 7(10) , 2195-201, (2009) A new domino autocatalytic reaction of imines with Meldrum's acid was described. In this reaction, a series of polycyclic spiro[5.5]undecane-1,5,9-trione and dispiro[4.2.5.2]pentadecane-9,13-dione derivatives, with remarkable diastereoselectivity, were succes... |
|
|
A novel one-pot three-(in situ five-)component condensation reaction: an unexpected approach for the synthesis of tetrahydro-2,4-dioxo-1H-benzo[b][1,5]diazepine-3-yl-2-methylpropanamide derivatives.
Org. Lett. 11(15) , 3342-5, (2009) A novel and efficient method has been developed for the synthesis of tetrahydro-2,4-dioxo-1H-benzo[b][1,5]diazepine-3-yl-2-methylpropanamide derivatives using an aromatic diamine, Meldrum's acid, and an isocyanide in CH(2)Cl(2) at ambient temperature in high ... |
|
|
Self-assembled Pd6 open cage with triimidazole walls and the use of its confined nanospace for catalytic Knoevenagel- and Diels-Alder reactions in aqueous medium.
Chemistry 18(39) , 12322-9, (2012) The two-component self-assembly of a 90° Pd(II) acceptor and a triimidazole donor led to the formation of a water-soluble semi-cylindrical cage with a hydrophobic cavity, which was separately crystallized with hydrophilic- and hydrophobic guests. The parent c... |
|
|
Electrochemical oxidation of catechols in the presence of phenyl-Meldrum's acid. Synthesis and kinetic evaluation.
Chem. Pharm. Bull. 58(1) , 23-6, (2010) Electrochemical oxidation of catechols in the presence of phenyl-Meldrum's acid as a nucleophile in aqueous solution has been studied in detail by means of cyclic voltammetry and controlled potential coulometry. The results indicate that the o-benzoquinone de... |
|
|
An efficient one-pot synthesis of pyrano[3,2-c]quinolin-2,5-dione derivatives catalyzed by L-proline.
Molecules 17(12) , 13856-63, (2012) A series of 4-aryl-6-methyl-3,4-dihydro-2H-pyrano[3,2-c]quinolin-2,5(6H)-diones were synthesized via the three-component reactions of aromatic aldehydes, 4-hydroxy-1-methylquinolin-2(1H)-one, and Meldrum's acid catalyzed by L-proline. The structures of the pr... |
|
|
Polymer synthesis: facilitating functionality.
Nature Chemistry 2(3) , 164-5, (2010)
|
|
|
Pseudo-five-component reaction between 3-formylchromones, meldrum's acid, isocyanides and primary arylamines: diversity-oriented synthesis of novel chromone-containing peptidomimetics.
ACS Comb. Sci. 13(6) , 659-66, (2011) An efficient and practical method has been developed for the diversity-oriented synthesis of chromone-containing tripeptides via pseudo-five-component reaction between 3-formylchromones, Meldrum's acid, isocyanides and primary aromatic amines for the generati... |
|
|
Catalytic enantioselective protonation of nitronates utilizing an organocatalyst chiral only at sulfur.
J. Am. Chem. Soc. 134(22) , 9058-61, (2012) The highly enantioselective protonation of nitronates formed upon the addition of α-substituted Meldrum's acids to terminally unsubstituted nitroalkenes is described. This work represents the first enantioselective catalytic addition of any type of nucleophil... |