Electrochemical oxidation of catechols in the presence of phenyl-Meldrum's acid. Synthesis and kinetic evaluation.
Davood Nematollahi, Maryam Bamzadeh, Hasan Shayani-Jam
文献索引:Chem. Pharm. Bull. 58(1) , 23-6, (2010)
全文:HTML全文
摘要
Electrochemical oxidation of catechols in the presence of phenyl-Meldrum's acid as a nucleophile in aqueous solution has been studied in detail by means of cyclic voltammetry and controlled potential coulometry. The results indicate that the o-benzoquinone derived from catechols participates in Michael addition reaction with phenyl-Meldrum's acid to form corresponding products. We derived some new "highly oxygenated compounds with catechol ring" with good yields based on electrochemical oxidation in the controlled potential condition in aqueous solutions, without toxic reagents and solvents at carbon electrode in an undivided cell, using an environmentally friendly method. Furthermore, the observed homogeneous rate constants (k(obs)) of the chemical reaction between o-benzoquinone and phenyl-Meldrum's acid were estimated by comparing the experimental cyclic voltammetric curves with the digitally simulated ones.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
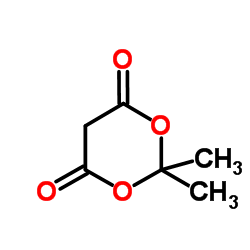 |
丙二酸环(亚)异丙酯
CAS:2033-24-1 |
C6H8O4 |
|
Synthesis and biological evaluation of arylidene analogues o...
2010-08-01 [Bioorg. Med. Chem. 18 , 5626-33, (2010)] |
|
One hundred years of Meldrum's acid: advances in the synthes...
2009-11-01 [Mol. Divers. 13(4) , 399-419, (2009)] |
|
A new domino autocatalytic reaction leading to polyfunctiona...
2009-05-21 [Org. Biomol. Chem. 7(10) , 2195-201, (2009)] |
|
A novel one-pot three-(in situ five-)component condensation ...
2009-08-06 [Org. Lett. 11(15) , 3342-5, (2009)] |
|
Self-assembled Pd6 open cage with triimidazole walls and the...
2012-09-24 [Chemistry 18(39) , 12322-9, (2012)] |