苯氧基乙酰氯
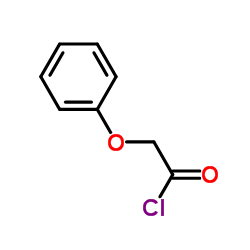
苯氧基乙酰氯结构式

|
常用名 | 苯氧基乙酰氯 | 英文名 | Phenoxyacetyl chloride |
|---|---|---|---|---|
| CAS号 | 701-99-5 | 分子量 | 170.593 | |
| 密度 | 1.2±0.1 g/cm3 | 沸点 | 226.3±13.0 °C at 760 mmHg | |
| 分子式 | C8H7ClO2 | 熔点 | 28 - 30ºC | |
| MSDS | 中文版 美版 | 闪点 | 108.3±0.0 °C | |
| 符号 |


GHS05, GHS07 |
信号词 | Danger |
|
Penicillin V acylase from Pectobacterium atrosepticum exhibits high specific activity and unique kinetics.
Int. J. Biol. Macromol. 79 , 1-7, (2015) Penicillin V acylases (PVAs, E.C.3.5.11) belong to the Ntn hydrolase super family of enzymes that catalyze the deacylation of the side chain from phenoxymethyl penicillin (penicillin V). Penicillin acylases find use in the pharmaceutical industry for the prod... |
|
|
Preparation and properties of 5-phenylphenoxymethylpenicillin.
J. Med. Chem. 18(5) , 486-90, (1975) Cycloaddition of azidoacetyl chloride to benzyl D-5,5-dimethyl-5-phenyl-2-thiazoline-4-carboxylate (1a) gave 5-phenyl-6alpha-azidopenicillanate (2a). By catalytic reduction of 2a and reaction with phenoxyacetyl chloride, 5-phenyl-6-epiphenoxymethylpenicillin ... |
|
|
Transient silylation of the guanosine O6 and amino groups facilitates N-acylation.
Org. Lett. 6(15) , 2555-7, (2004) [reaction: see text] The formation of a guanosine derivative silylated at both the O6 and amino groups was identified by (15)N NMR. This intermediate allows facile reaction with acetyl chloride or phenoxyacetyl chloride to give in high yield the corresponding... |
|
|
Synthesis and antimicrobial evaluation of novel bis-β-lactam grafted macrocycles.
Med. Chem. , (2014) A series of macrocyclic bis-β-lactams has been synthesized in three good yielding steps using a Staudinger [2+2] cycloaddition reaction of ketene derived from phenoxyacetyl chloride as the key step. The reaction provided a diastereomeric mixture of cis-anti-c... |
|
|
Tetrahedron 63 , 3380, (2007)
|
|
|
Tetrahedron Lett. 48 , 1657, (2007)
|