Organic Letters
2004-07-22
Transient silylation of the guanosine O6 and amino groups facilitates N-acylation.
Yupeng Fan, Barbara L Gaffney, Roger A Jones
文献索引:Org. Lett. 6(15) , 2555-7, (2004)
全文:HTML全文
摘要
[reaction: see text] The formation of a guanosine derivative silylated at both the O6 and amino groups was identified by (15)N NMR. This intermediate allows facile reaction with acetyl chloride or phenoxyacetyl chloride to give in high yield the corresponding N-protected guanosine derivatives, suitable for use in RNA synthesis. The acetyl and phenoxyacetyl amino protecting groups are, respectively, 4 and 230 times more labile than the isobutyryl group to methylamine/ethanol deprotection.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
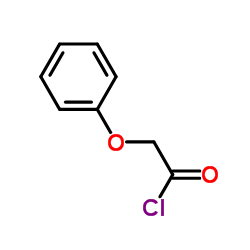 |
苯氧基乙酰氯
CAS:701-99-5 |
C8H7ClO2 |
相关文献:
更多...
|
Penicillin V acylase from Pectobacterium atrosepticum exhibi...
2015-08-01 [Int. J. Biol. Macromol. 79 , 1-7, (2015)] |
|
Preparation and properties of 5-phenylphenoxymethylpenicilli...
1975-05-01 [J. Med. Chem. 18(5) , 486-90, (1975)] |
|
Synthesis and antimicrobial evaluation of novel bis-β-lactam...
2014-01-01 [Med. Chem. , (2014)] |
|
[Tetrahedron 63 , 3380, (2007)] |
|
[Tetrahedron Lett. 48 , 1657, (2007)] |