马立马司他
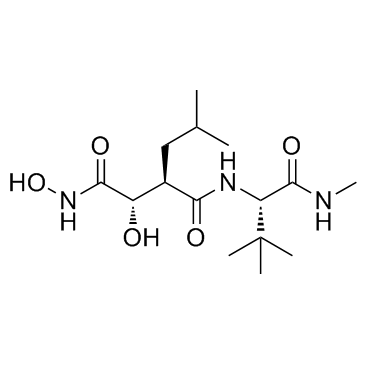
马立马司他结构式

|
常用名 | 马立马司他 | 英文名 | Marimastat (BB-2516) |
|---|---|---|---|---|
| CAS号 | 154039-60-8 | 分子量 | 331.408 | |
| 密度 | 1.1±0.1 g/cm3 | 沸点 | N/A | |
| 分子式 | C15H29N3O5 | 熔点 | 148℃ | |
| MSDS | 中文版 美版 | 闪点 | N/A |
|
Nestin depletion induces melanoma matrix metalloproteinases and invasion.
Lab. Invest. 94(12) , 1382-95, (2014) Matrix metalloproteinases (MMPs) are key biological mediators of processes as diverse as wound healing, embryogenesis, and cancer progression. Although MMPs may be induced through multiple signaling pathways, the precise mechanisms for their regulation in can... |
|
|
Interleukin 21 and Its Receptor Play a Role in Proliferation, Migration and Invasion of Breast Cancer Cells.
Cancer Genomics Proteomics 12 , 211-21, (2015) Interleukin 21 (IL21) is a cytokine produced predominantly by cluster of differentiation 4 (CD4+) T-cells and natural killer T-cells. There exists evidence that IL21 is implicated in various immunological processes through its specific receptor (IL21R). Howev... |
|
|
Log-scale dose response of inhibitors on a chip.
Anal. Chem. 83(16) , 6148-53, (2011) We demonstrate the accommodation of log-scale concentration gradients of inhibitors on a single microfluidic chip with a semidirect dilution capability of reagents for the determination of the half-inhibitory concentration or IC(50). The chip provides a uniqu... |
|
|
Matrix Metalloproteinase Inhibition as a Novel Anticancer Strategy: A Review with Special Focus on Batimastat and Marimastat
Pharmacol. Ther. 75(1) , 69-75, (1997) Matrix metalloproteinases (MMPs) are a homologous family of enzymes that are involved in tissue remodeling and morphogenesis. Collectively, these enzymes are capable of degrading all components of the extracellular matrix, and they play an important role in n... |
|
|
Marimastat: the clinical development of a matrix metalloproteinase inhibitor.
Expert Opin. Investig. Drugs 9(12) , 2913-22, (2000) Marimastat (BB-2516) is the first orally bioavailable matrix metalloproteinase inhibitor to have entered clinical trials in the field of oncology. It has excellent bioavailability and has completed Phase I, II and some Phase III trials. In Phase I studies, wh... |
|
|
Plasma matrix metalloproteinases 7 and 9 in patients with metastatic breast cancer treated with marimastat or placebo: Eastern Cooperative Oncology Group trial E2196.
Clin. Breast Cancer 6(6) , 525-9, (2006) The purpose of this study was to evaluate the prognostic and predictive utility of measuring plasma levels of matrix metalloproteinase (MMP)-7 and MMP-9 in patients with metastatic breast cancer (MBC) treated with the oral MMP inhibitor (MMPI) marimastat or a... |
|
|
A novel twist in polycystic liver disease.
Gut 63(10) , 1533-4, (2014)
|
|
|
TNF-alpha as a target for anti-asthma therapy.
Clin. Sci. 110(2) , 265, (2006)
|
|
|
Marimastat: BB 2516, TA 2516.
Drugs R. D. 4(3) , 198-203, (2003) Marimastat [BB 2516, TA 2516] is a second-generation anticancer drug originally developed with British Biotech in Europe and North America. It is an orally active metalloprotease inhibitor of the same class as batimastat, and is the first compound in this cla... |
|
|
The importance of estimating the therapeutic index in the development of matrix metalloproteinase inhibitors.
Cardiovasc. Res. 69(3) , 677-87, (2006) At least 56 matrix metalloproteinase (MMP) inhibitors have been pursued as clinical candidates since the late 1970's when the first drug discovery program targeting this enzyme family began. Some of these clinical candidates were pursued for multiple indicati... |