Plasma matrix metalloproteinases 7 and 9 in patients with metastatic breast cancer treated with marimastat or placebo: Eastern Cooperative Oncology Group trial E2196.
Stanley Zucker, Molin Wang, Joseph A Sparano, William J Gradishar, James N Ingle, Nancy E Davidson
文献索引:Clin. Breast Cancer 6(6) , 525-9, (2006)
全文:HTML全文
摘要
The purpose of this study was to evaluate the prognostic and predictive utility of measuring plasma levels of matrix metalloproteinase (MMP)-7 and MMP-9 in patients with metastatic breast cancer (MBC) treated with the oral MMP inhibitor (MMPI) marimastat or a placebo.We measured plasma levels of MMP-7 and MMP-9 using an enzyme-linked immunoassay method in 140 evaluable patients with MBC enrolled on a multicenter clinical trial that compared the MMPI marimastat with placebo. Specimens were collected after completion of first-line chemotherapy in patients who had responding or stable disease and 3, 6, and 12 months after initiation of marimastat (10 mg orally twice daily) or placebo.Baseline plasma MMP-7 and MMP-9 levels did not correlate with each other or with progression-free or overall survival. In addition, serial evaluation of plasma MMP-7 and MMP-9 levels revealed no significant changes over time in the marimastat or placebo groups or any correlation with trough plasma marimastat level.We conclude that measurement of plasma MMP-7 or MMP-9 levels, as performed in our trial, was not a useful prognostic or predictive factor in patients with MBC or in patients treated with an MMPI.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
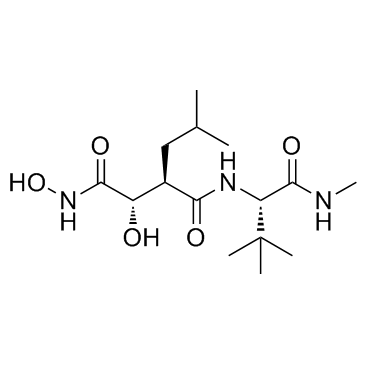 |
马立马司他
CAS:154039-60-8 |
C15H29N3O5 |
|
Nestin depletion induces melanoma matrix metalloproteinases ...
2014-12-01 [Lab. Invest. 94(12) , 1382-95, (2014)] |
|
Interleukin 21 and Its Receptor Play a Role in Proliferation...
2015-01-01 [Cancer Genomics Proteomics 12 , 211-21, (2015)] |
|
Log-scale dose response of inhibitors on a chip.
2011-08-15 [Anal. Chem. 83(16) , 6148-53, (2011)] |
|
Matrix Metalloproteinase Inhibition as a Novel Anticancer St...
1997-01-01 [Pharmacol. Ther. 75(1) , 69-75, (1997)] |
|
Marimastat: the clinical development of a matrix metalloprot...
2000-12-01 [Expert Opin. Investig. Drugs 9(12) , 2913-22, (2000)] |