四氟对苯醌
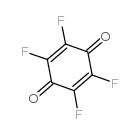
四氟对苯醌结构式

|
常用名 | 四氟对苯醌 | 英文名 | tetrafluoro-1,4-benzoquinone |
|---|---|---|---|---|
| CAS号 | 527-21-9 | 分子量 | 180.05700 | |
| 密度 | 1.62 g/cm3 | 沸点 | 133.1ºC at 760 mmHg | |
| 分子式 | C6F4O2 | 熔点 | 183-186 °C (subl.)(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 44.6ºC | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Cytochrome P450-mediated oxidation of pentafluorophenol to tetrafluorobenzoquinone as the primary reaction product.
Chem. Res. Toxicol. 6(5) , 674-80, (1993) In the present study the oxidative dehalogenation of a para-halogenated phenol was studied using pentafluorophenol and its non-para-halogenated analogue 2,3,5,6-tetrafluorophenol as model compounds. 19F NMR was used to characterize the metabolite patterns. In... |
|
|
Detection and mechanistic investigation of halogenated benzoquinone induced DNA damage by photoelectrochemical DNA sensor.
Anal. Bioanal. Chem 397(6) , 2395-400, (2010) Halogenated phenols are widely used as biocides and are considered to be possibly carcinogenic to humans. In this report, a previously developed photoelectrochemical DNA sensor was employed to investigate DNA damage induced by tetra-halogenated quinones, the ... |
|
|
Molecular mechanism for metal-independent production of hydroxyl radicals by hydrogen peroxide and halogenated quinones.
Proc. Natl. Acad. Sci. U. S. A. 104(45) , 17575-8, (2007) We have shown previously that hydroxyl radicals (HO*) can be produced by H2O2 and halogenated quinones, independent of transition metal ions; however, the underlying molecular mechanism is still unclear. In the present study, using the electron spin resonance... |
|
|
Self-promoted electron transfer from cobalt(II) porphyrin to p-fluoranil to produce a dimer radical anion-cobalt(III) porphyrin complex.
J. Am. Chem. Soc. 125(41) , 12416-7, (2003) Self-promoted electron transfer from a cobalt(II) porphyrin [Co(II)OEP] to p-fluoranil (F4Q) occurs, exhibiting a second-order dependence of the electron-transfer rate with respect to the F4Q concentration due to the formation of a strong complex between the ... |
|
|
A convenient method for the preparation of symmetrical or unsymmetrical ethers by the coupling of two alcohols via a new type of oxidation-reduction condensation using tetrafluoro-1, 4-benzoquinone. Shintou T and Mukaiyama T.
Chem. Lett. 11 , 984-985, (2003)
|
|
|
Complexation Behavior of Binaphthol/Tetrafluoro-1, 4-benzoquinone Charge-Transfer Complex. Imai Y, et al.
Cryst. Growth Des. 9(5) , 2393-2397, (2009)
|
|
|
Generation and spectroscopic characterization of the 2, 3, 5, 6-tetramethoxy-1, 4-benzosemiquinone reactive intermediate. Mattar SM, et al.
Chem. Phys. Lett. 352(1) , 39-47, (2002)
|