tetrafluoro-1,4-benzoquinone
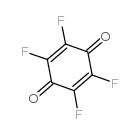
tetrafluoro-1,4-benzoquinone structure
|
Common Name | tetrafluoro-1,4-benzoquinone | ||
|---|---|---|---|---|
| CAS Number | 527-21-9 | Molecular Weight | 180.05700 | |
| Density | 1.62 g/cm3 | Boiling Point | 133.1ºC at 760 mmHg | |
| Molecular Formula | C6F4O2 | Melting Point | 183-186 °C (subl.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 44.6ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 2,3,5,6-tetrafluorocyclohexa-2,5-diene-1,4-dione |
|---|---|
| Synonym | More Synonyms |
| Density | 1.62 g/cm3 |
|---|---|
| Boiling Point | 133.1ºC at 760 mmHg |
| Melting Point | 183-186 °C (subl.)(lit.) |
| Molecular Formula | C6F4O2 |
| Molecular Weight | 180.05700 |
| Flash Point | 44.6ºC |
| Exact Mass | 179.98300 |
| PSA | 34.14000 |
| LogP | 1.43940 |
| Index of Refraction | 1.409 |
| InChIKey | JKLYZOGJWVAIQS-UHFFFAOYSA-N |
| SMILES | O=C1C(F)=C(F)C(=O)C(F)=C1F |
| Stability | Stable. Non-flammable. Incompatible with strong oxidizing agents, alkali metals, strong reducing agents. |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2914700090 |
| Precursor 7 | |
|---|---|
| DownStream 9 | |
| HS Code | 2914700090 |
|---|---|
| Summary | HS: 2914700090 halogenated, sulphonated, nitrated or nitrosated derivatives of ketones and quinones, whether or not with other oxygen function Tax rebate rate:9.0% Supervision conditions:none VAT:17.0% MFN tariff:5.5% General tariff:30.0% |
|
Cytochrome P450-mediated oxidation of pentafluorophenol to tetrafluorobenzoquinone as the primary reaction product.
Chem. Res. Toxicol. 6(5) , 674-80, (1993) In the present study the oxidative dehalogenation of a para-halogenated phenol was studied using pentafluorophenol and its non-para-halogenated analogue 2,3,5,6-tetrafluorophenol as model compounds. 1... |
|
|
Detection and mechanistic investigation of halogenated benzoquinone induced DNA damage by photoelectrochemical DNA sensor.
Anal. Bioanal. Chem 397(6) , 2395-400, (2010) Halogenated phenols are widely used as biocides and are considered to be possibly carcinogenic to humans. In this report, a previously developed photoelectrochemical DNA sensor was employed to investi... |
|
|
Molecular mechanism for metal-independent production of hydroxyl radicals by hydrogen peroxide and halogenated quinones.
Proc. Natl. Acad. Sci. U. S. A. 104(45) , 17575-8, (2007) We have shown previously that hydroxyl radicals (HO*) can be produced by H2O2 and halogenated quinones, independent of transition metal ions; however, the underlying molecular mechanism is still uncle... |
| EINECS 208-411-9 |
| Tetrafluoroquinone |
| MFCD00001592 |
| p-Fluoranil |
| Tetrafluoro-p-benzoquinone |
| p-Benzoquinone,2,3,5,6-tetrafluoro |
| 1,2,4,5-tetrafluoro-p-benzoquinone |
| o-fluoranil |
| Tetrafluoro-1,4-benzoquinone |
| Fluoroanil |
| Fluoranil |
 CAS#:771-63-1
CAS#:771-63-1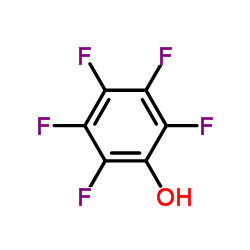 CAS#:771-61-9
CAS#:771-61-9 CAS#:105608-97-7
CAS#:105608-97-7 CAS#:105608-98-8
CAS#:105608-98-8 CAS#:880-78-4
CAS#:880-78-4 CAS#:118-75-2
CAS#:118-75-2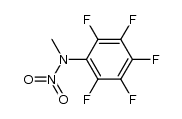 CAS#:370556-45-9
CAS#:370556-45-9 CAS#:355-02-2
CAS#:355-02-2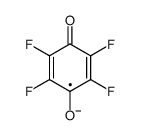 CAS#:42439-31-6
CAS#:42439-31-6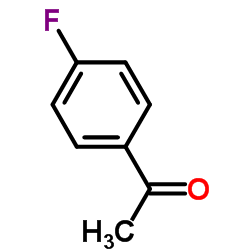 CAS#:403-42-9
CAS#:403-42-9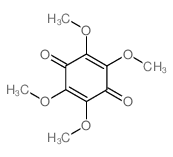 CAS#:3117-06-4
CAS#:3117-06-4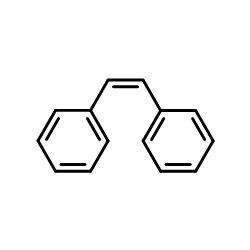 CAS#:645-49-8
CAS#:645-49-8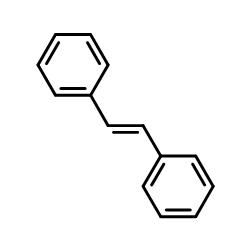 CAS#:103-30-0
CAS#:103-30-0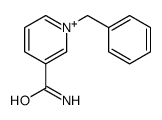 CAS#:16183-83-8
CAS#:16183-83-8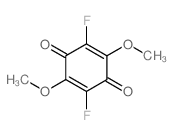 CAS#:653-41-8
CAS#:653-41-8
